Edit Once, Lower for Life: PCSK9 and the Longevity Bet
In 2025, the first human data for in vivo PCSK9 base editing showed large, durable LDL-C drops after a single infusion. If safety holds and access follows, one-and-done edits could shift heart disease prevention from chronic care to permanent risk cut.
The one-shot cholesterol edit is no longer sci‑fi
Something fundamental changed this year. In April 2025, early human data showed that a single intravenous dose of an in vivo base‑editing medicine targeting PCSK9 produced large, dose‑dependent drops in LDL cholesterol, with an average reduction above 50 percent at higher doses and a maximum near 70 percent. Those results came from Verve’s Heart‑2 study of VERVE‑102, and the safety profile looked clean in the first 14 participants. For the first time, a one‑and‑done genetic edit in the liver lowered the most important modifiable driver of atherosclerosis in humans after a single treatment, not a daily pill or twice‑yearly shot. Verve reported the dataset on April 14, 2025.
Three months later, the story got louder. On July 25, 2025, Eli Lilly closed its acquisition of Verve, signaling that a big company with scale intends to push single‑dose cardiovascular gene editing through late‑stage studies and, if it works, into routine care. Lilly framed the deal as a path to “lifelong cardiovascular risk reduction” with a single course of therapy. That is the right way to think about it. A durable edit is not just another cholesterol drug. It is a new class of preventive medicine. Lilly’s completion announcement on July 25, 2025 made the case explicit.
PCSK9 101 and why LDL‑C is the lever on lifespan
PCSK9 is a protein that tags LDL receptors in the liver for degradation. Fewer receptors on hepatocytes means less LDL cholesterol pulled from the blood. People born with PCSK9 loss‑of‑function variants tend to have strikingly low LDL‑C and very low lifetime rates of coronary disease, which is the genetic hint that switching PCSK9 off is cardio‑protective. That biology has already reshaped treatment with monoclonal antibodies and siRNA targeting PCSK9. Base editing aims upstream: instead of neutralizing the protein again and again, it changes one DNA letter in the PCSK9 gene in hepatocytes so the gene stays off.
VERVE‑102 uses a lipid nanoparticle to deliver two RNAs to the liver. One RNA encodes an adenine base editor. The other is a guide that directs the editor to PCSK9. The editor converts a single base in a splice site, which prevents normal PCSK9 production. Because hepatocytes continually renew, the decisive question is durability. If enough cells are edited and the edit persists through cell turnover, the LDL‑C reduction should be long‑lived.
What the 2025 human data actually show
Heart‑2 is an open‑label, ascending‑dose Phase 1b in patients with heterozygous familial hypercholesterolemia or premature coronary artery disease who need more LDL‑C lowering. As of the April 14 cut, 14 participants had at least 28 days of follow‑up across three dose levels. The signals were dose‑dependent. In the 0.6 mg/kg cohort, the mean LDL‑C reduction was reported at roughly 53 percent with a maximum of 69 percent after one infusion. Blood PCSK9 protein fell in parallel.
On safety, no treatment‑related serious adverse events were reported among those 14 participants, and the typical hepatic and hematologic labs did not show clinically significant issues in that initial window. Enrollment has since moved into a 0.7 mg/kg cohort, with final dose‑escalation and durability data expected in the second half of 2025. The U.S. FDA cleared an IND for Phase 2 to begin in the second half of the year, now under Lilly’s umbrella. It is early, but as first human evidence of in vivo base editing that changes a major cardiometabolic risk factor, this is a landmark.
From chronic management to permanent risk reduction
Cardiovascular risk accumulates with time and LDL exposure. That is why sequence matters. An intervention that knocks LDL‑C down by 50 to 60 percent for decades should reduce events far more than the same reduction applied inconsistently over the same span. Real‑world adherence to statins is not great. Many people stop within a year. Even the best PCSK9 injectables require ongoing dosing and follow‑up. A one‑time edit replaces adherence with biology. If the edit is safe and durable, the area under the LDL‑C curve collapses for the rest of a patient’s life.
Translating early pharmacodynamics into hard outcomes requires caution. Regulators accept LDL‑C as a validated surrogate for benefit in high‑risk groups, but approval for broad secondary prevention may still hinge on cardiovascular outcomes. The optimistic projection looks like this: if a single dose produces a stable 50 to 60 percent LDL‑C reduction for many years, major adverse cardiovascular events should fall substantially, and the absolute benefit compounds with time because exposure drops permanently. The conservative projection is that durability and safety may limit use to the highest‑risk patients until outcomes are unequivocal.
Safety is the gating factor
The field learned hard lessons in 2024 when an earlier construct, VERVE‑101, saw a serious drug‑related adverse event and enrollment was paused. VERVE‑102 uses the same base editor and guide but a different lipid nanoparticle that includes a GalNAc ligand to more selectively target hepatocytes via the asialoglycoprotein receptor and a different ionizable lipid with a better clinical track record. That change, plus meticulous premedication, likely explains why the early Heart‑2 safety readout is cleaner. But Phase 1b is small and brief. A few key questions have to be answered with larger, longer datasets:
- Off‑target editing. Base editors are more precise than nuclease cutting, but bystander edits and rare off‑targets remain possible. Deep sequencing of liver biopsies and cell‑free DNA helps, yet it will take time to correlate any low‑frequency edits with real‑world consequences.
- On‑target but unintended effects. Editing a splice site turns PCSK9 off as designed, but any unexpected splicing artifacts or cryptic transcripts must be excluded.
- Immunogenicity. The editor is translated from mRNA and is present transiently, which should limit immune issues, but prior exposure to bacterial proteins and repeated LNP exposure can matter. One benefit of a one‑shot edit is the lack of chronic re‑exposure.
- Hepatic safety. Transient liver enzyme bumps are common with LNP therapies. The 2025 dataset did not show clinically significant ALT, AST, bilirubin, or platelet issues in the first 14 participants. Larger trials must confirm that profile across broader comorbidities.
Expect U.S. long‑term follow‑up requirements typical for in vivo gene therapies, often up to 15 years. That is appropriate for a permanent edit, and it means registries, post‑marketing surveillance, and biopsy substudies are not optional extras. They are the product.
How it stacks up against statins and PCSK9 drugs
- Statins: 30 to 50 percent LDL‑C reduction depending on intensity, proven mortality benefit, low cost, and they remain first‑line. The problem is adherence and intolerance in a minority of patients. Real‑world discontinuation is common, which erodes the theoretical benefit.
- PCSK9 monoclonal antibodies: roughly 60 percent LDL‑C reduction on top of statins, strong outcomes data, injections every 2 to 4 weeks, and list prices that have dropped but still require prior authorization hurdles. Adherence is better than pills for some, worse for others.
- Inclisiran (PCSK9 siRNA): about 50 percent LDL‑C reduction with twice‑yearly dosing after a loading schedule. Simpler logistics, but still a maintenance therapy.
A successful PCSK9 edit needs to match or beat the LDL‑C lowering of antibodies and siRNA, with a safety profile that withstands chronic use comparisons. The differentiator is durability. If a single infusion maintains a 50 to 60 percent LDL‑C cut for many years without late surprises, the value proposition is powerful for patients, payers, and public health.
The next edits: Lp(a) and ANGPTL3
LDL‑C is necessary but not sufficient. Two other lipid‑related drivers matter for many patients:
- Lipoprotein(a), or Lp(a). It is mostly genetic, barely moves with lifestyle, and adds risk for coronary disease, stroke, and aortic stenosis. Multiple RNA‑based drugs are in late stages to lower Lp(a), and Lilly has an every‑few‑months siRNA that cut Lp(a) by more than 90 percent in a mid‑stage study. Verve nominated VERVE‑301, a liver‑targeted in vivo editor designed to permanently turn off the LPA gene. If an edit can safely drive a large, lasting Lp(a) drop, it could complement LDL‑C control in the highest‑risk patients whose events persist despite perfect LDL‑C.
- ANGPTL3. This hepatocyte‑secreted protein regulates triglyceride‑rich lipoproteins and LDL‑C. Inactivating ANGPTL3 lowers remnant cholesterol and LDL‑C, which is relevant for mixed dyslipidemia, refractory hypercholesterolemia, and homozygous FH. Verve’s VERVE‑201, an ANGPTL3 base‑editing candidate, entered Phase 1b in late 2024 with a program update slated for the second half of 2025.
These targets point to a future stack of one‑time edits that address three independent levers of atherosclerosis: LDL‑C, Lp(a), and remnant cholesterol. The sequencing of those edits will be clinical, not genomic. Many patients may only need PCSK9. A subset with very high Lp(a) could eventually receive both, ideally with careful spacing and lifetime monitoring.
Regulators and payers will shape the slope of adoption
Regulators have two hard jobs here. First, ensure safety for an irreversible intervention. Second, decide how much LDL‑C lowering and how much durability is enough for approval in specific populations. Expect initial labels to focus on heterozygous FH and very high‑risk secondary prevention, where the benefit‑risk trade is most favorable and LDL‑C is a validated surrogate. Outcomes data would then enable broader use.
Payers face a different calculus. One‑time therapies are usually expensive upfront. There will be pressure to align price with a present value of decades of avoided events and procedures. Expect outcomes‑based contracts, milestone payments over time, and requirements for documented on‑target LDL‑C lowering. Because the treatment is a brief infusion with short monitoring, capacity is easier than for cell therapies, but prior authorization criteria will be tight at first.
Access also hinges on identification. A one‑time PCSK9 edit pays off most for people with high lifetime exposure to LDL‑C. That argues for broader lipid screening earlier in life, reflex testing for Lp(a), and proactive identification of familial hypercholesterolemia through cascade screening. If we want population‑scale prevention, we have to find the right populations early.
Why this could be the first true preventive longevity medicine at scale
Most longevity talk stays abstract. This is concrete. A safe, durable PCSK9 edit shifts prevention from behavior and adherence to physiology and time. It compresses risk for the single biggest killer by permanently lowering the causal factor. It does not require a specialized surgical suite. It uses manufacturing and delivery technologies already proven at pandemic scale for mRNA and LNPs. It targets a common biology in the liver, not a rare mutation in an exotic tissue.
The hurdle is not imagination. It is evidence. The 2025 Heart‑2 data say we can do the edit safely in the near term and get the LDL‑C drop we want. The next two years will tell us about durability at scale, class‑wide safety, and how regulators want to see outcomes. If those dominoes fall, the uptake could look more like the adoption of statins in the 1990s than a boutique gene therapy.
What to watch next
- Final Heart‑2 dose‑escalation and early durability data in the second half of 2025, including the 0.7 mg/kg cohort.
- Initiation and early readouts from Phase 2 in 2025 to 2026, with U.S. sites enrolling.
- ANGPTL3 program updates for VERVE‑201 and progress toward first‑in‑human for the Lp(a) editor VERVE‑301.
- Safety database growth, including systematic off‑target assessments and liver safety across comorbid populations.
- Regulatory guidance on surrogate endpoints for approval in high‑risk groups and the design of outcomes studies for broader labels.
- Payer pilots for outcomes‑based payments and real‑world registries to track long‑term benefit and rare risks.
Bottom line
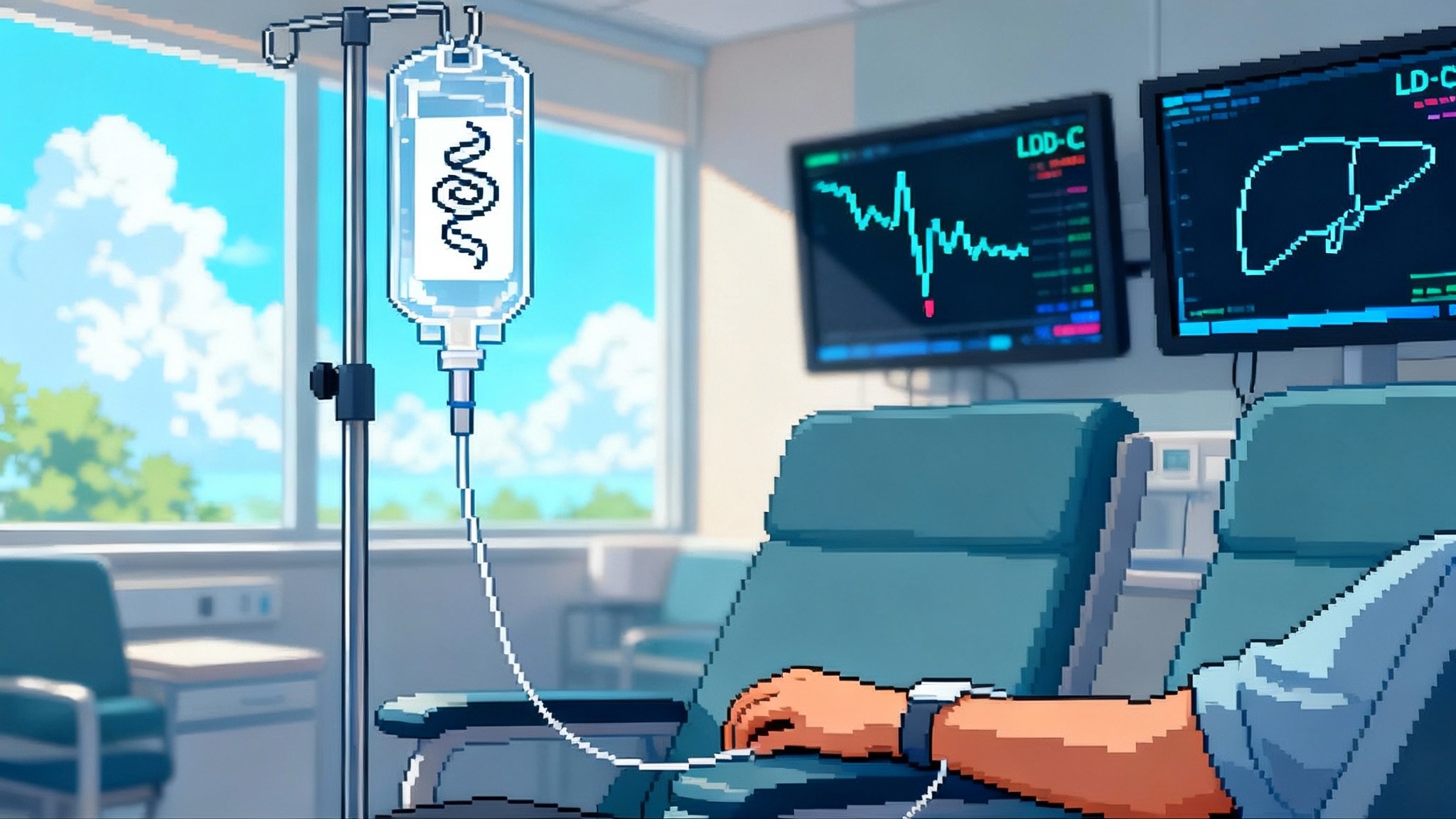
If the edit is safe and durable, a single session in an infusion chair could replace decades of pills and injections for millions of people at risk of heart attack and stroke. That is not just convenience. It is a structural shift in how we extend healthy life at population scale. The 2025 human data and Lilly’s move to industrialize the program turn that possibility into a credible path. Now the field has to do the hard work of proving it, building access around it, and earning the trust to deploy it widely.