Longevity
Longevity
Articles under the Longevity category.
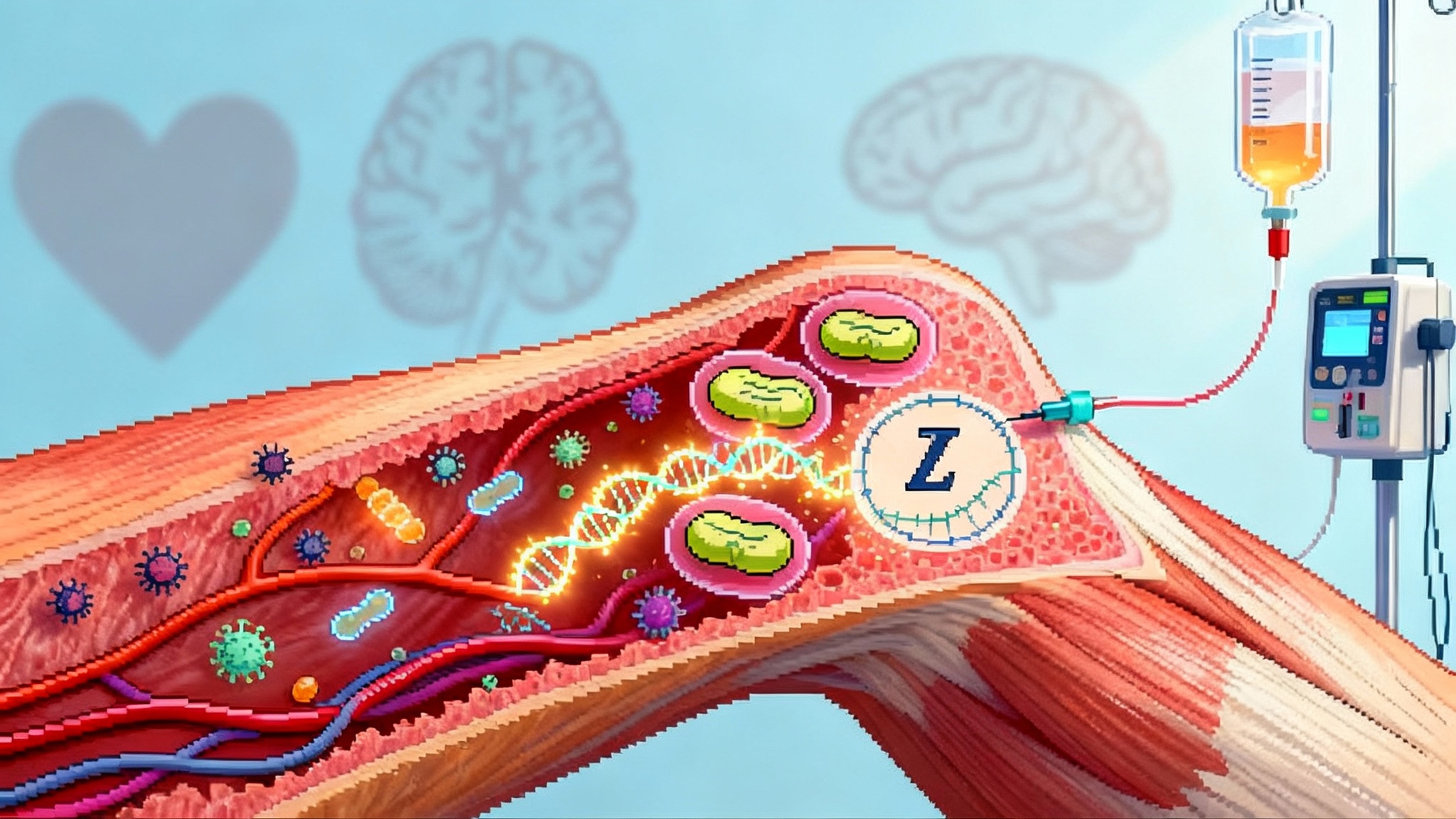
FDA’s first mitochondria drug and the longevity playbook
The FDA’s September 2025 accelerated approval of Forzinity, the first mitochondria-targeted therapy, is a turning point. Here is how cardiolipin repair could scale from a rare disease win to near-term trials in aging muscles, hearts, and retinas—and what endpoints and biomarkers will actually matter.
Pets take point: 2025’s leap in longevity drug approvals
In 2025, a weekly sirolimus tablet for cats won conditional FDA approval and a senior-dog longevity pill cleared a key efficacy review. Pet medicine is becoming the proving ground for geroprotectors, and this blueprint shows how it translates to humans.
GLP-1s After Weight Loss: The New Longevity Infrastructure
In 2025, GLP-1 drugs move beyond weight loss into validated risk reduction. Regulators, outcomes trials, and insurers now point to survival and organ protection as the main story.
Klotho gene therapy’s 2025 cross‑over to first‑in‑human
A 2025 Molecular Therapy study reported that a single AAV dose elevating secreted Klotho extended male mouse lifespan by roughly 15 to 20 percent and improved aging markers. Here is a concrete, risk-aware plan to reach early human trials, with routes, biomarkers, and go or no go gates.
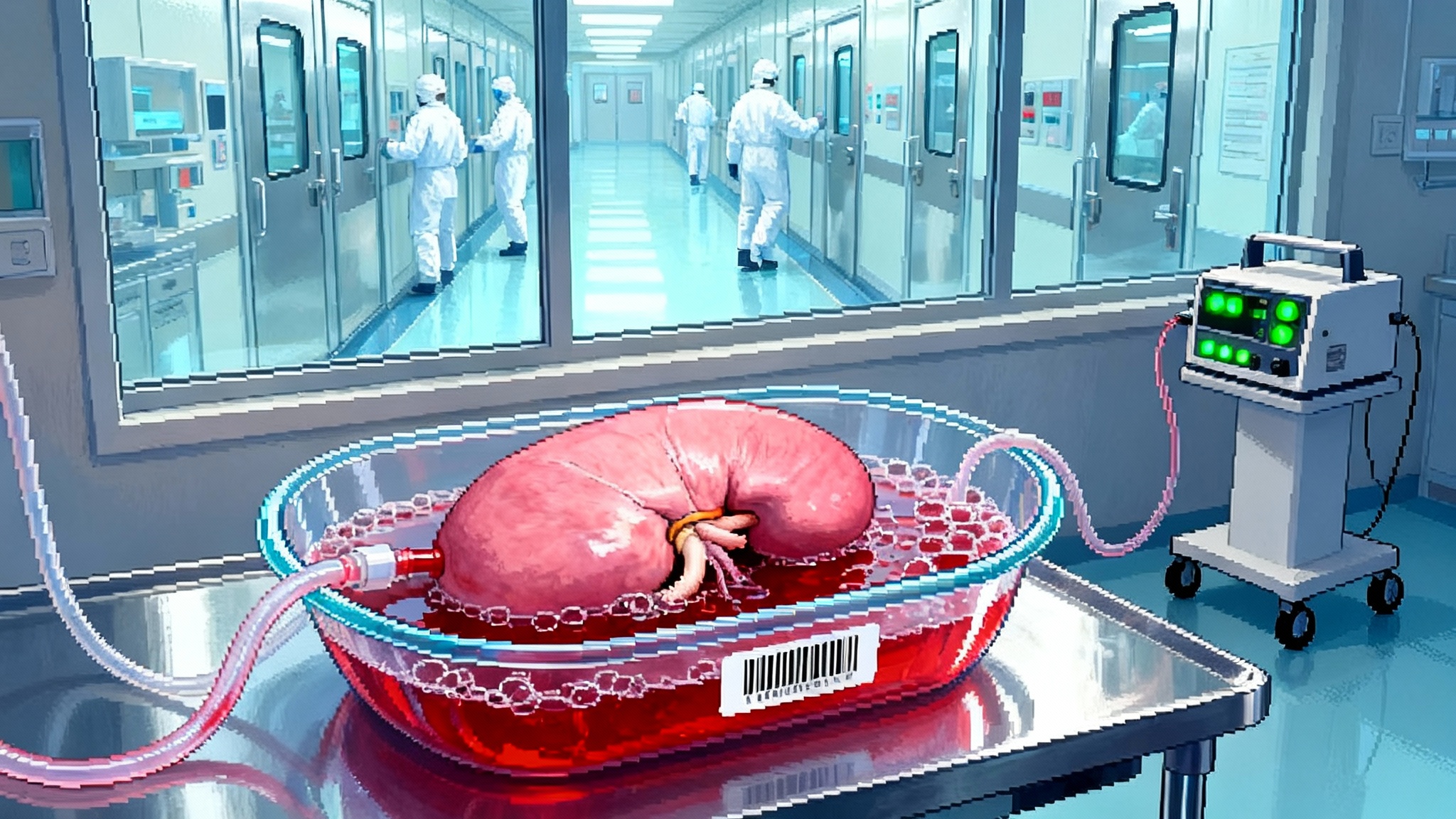
Gene Edited Pig Kidneys Enter First U.S. Clinical Trials in 2025
The FDA has cleared the first U.S. human trials of gene-edited pig kidneys. We unpack the 10 to 69 genomic edits, the new anti-rejection regimens, and the biosecure pig herds that could finally end the kidney shortage.
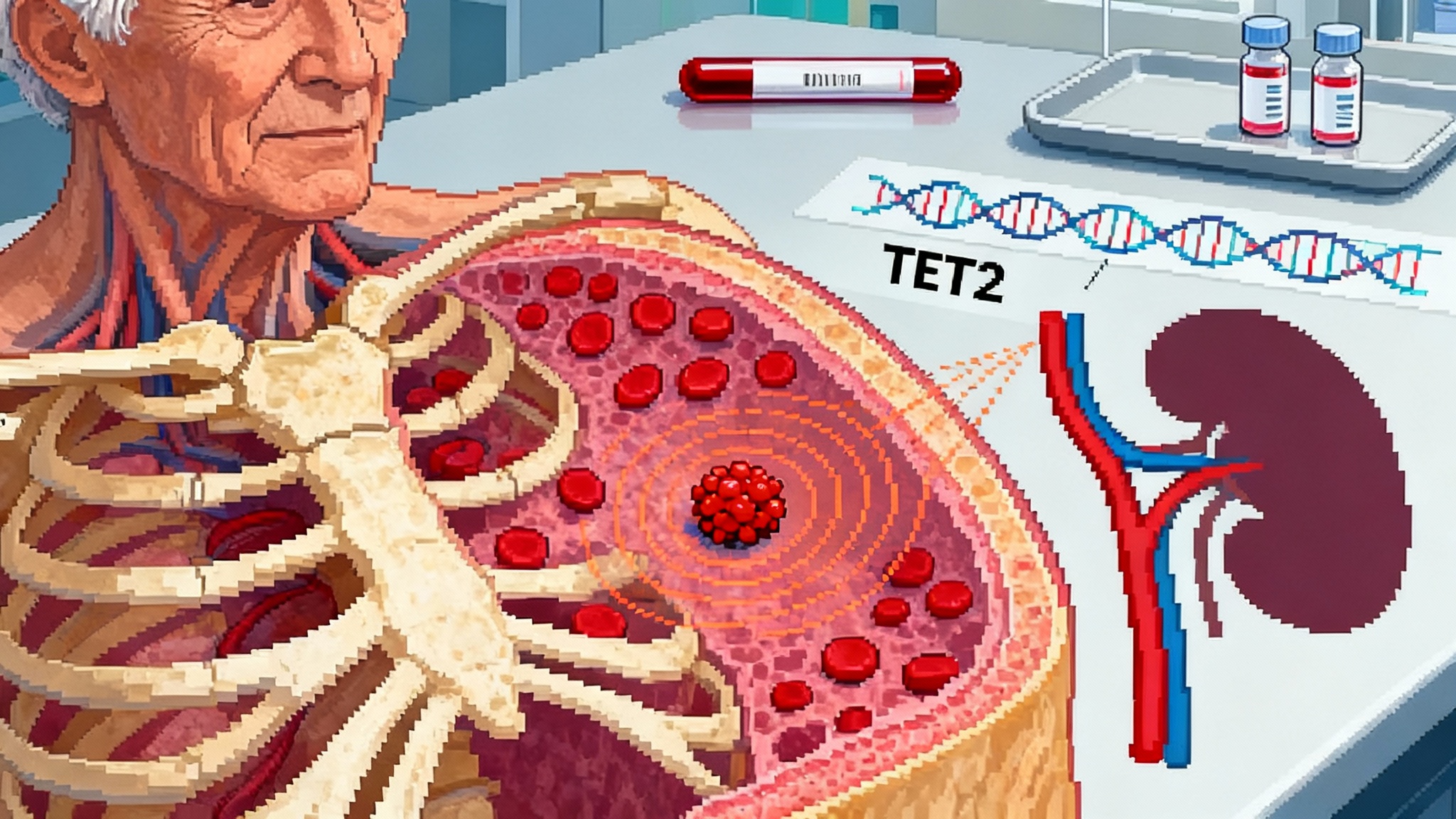
CHIP Goes Therapeutic: 2025’s Shift From Marker to Target
ESC 2025 data show low-dose colchicine can slow TET2-driven clonal growth, while new mega-cohort analyses pinpoint who is at risk. Here is why routine CHIP screening is near and how a 12–24 month rollout could work.
PCSK9 base editing and primary prevention rewrite LDL care
Two signals in 2025 mark a new era in cholesterol care. AHA VESALIUS-CV shows PCSK9 inhibitors prevent first cardiovascular events, and early Heart-2 data for VERVE-102 suggests single-infusion, durable LDL reductions following Lilly’s Verve acquisition.
FDA Reinstates NMN: A 24-month Plan to Prove NAD+ Works
After the FDA’s September 2025 clarification that NMN is lawful in supplements, the NAD+ space has a narrow window to trade sizzle for substance. This roadmap lays out the evidence plan, quality playbook, and retail relisting strategy needed to make NAD repletion real.
Glycation’s 2025 Pivot: Anti-glycation Enters Trials
Mouse data put anti-glycation upstream of multiple hallmarks. Here is what Gly-Low changes, how to design a human trial, what to measure now, and the milestones to watch in 2026.
Nanowarming Brings Organ Banking to Human Scale in 2025
Two milestones in 2025 moved organ cryopreservation from elegant physics to practical engineering: a 10-day pig kidney transplant after cryostorage and liter-scale nanowarming that heats human-sized volumes uniformly. Here is what changed, why it matters, and what comes next.
Thymus Revival 2025: Turning Immune Aging into Treatable Biology
A wave of 2025 breakthroughs is pushing thymus preservation and regeneration from lab to clinic. Here is why immunosenescence is the next big lever for healthspan, what to watch over the next year, and how to fast-track the right biomarkers.
Plasma Exchange's First RCT Bends Human Biological Age
A May 2025 single-blind randomized trial from the Buck Institute and Circulate Health reports that therapeutic plasma exchange, especially when paired with intravenous immunoglobulin, shifted multi-omics biological age in older adults. Here is what changed, why it matters, and how this could enter clinics without outrunning the evidence.
Mitochondrial Editing’s 2025 Leap to Injectable Care
Mitochondrial base editing moved from clever biology to an injectable therapeutic candidate in 2025. See the new editors, in vivo delivery data, and a pragmatic first-in-human plan aimed at aging tissues.
From Skin to Egg: IVG’s 2025 Breakthrough and What Comes Next
In September 2025, OHSU researchers reported human eggs made from skin cells and then fertilized in vitro. This review explains what was achieved, why it could extend reproductive longevity, the safety and regulatory gates ahead, and a realistic path to first pilots.
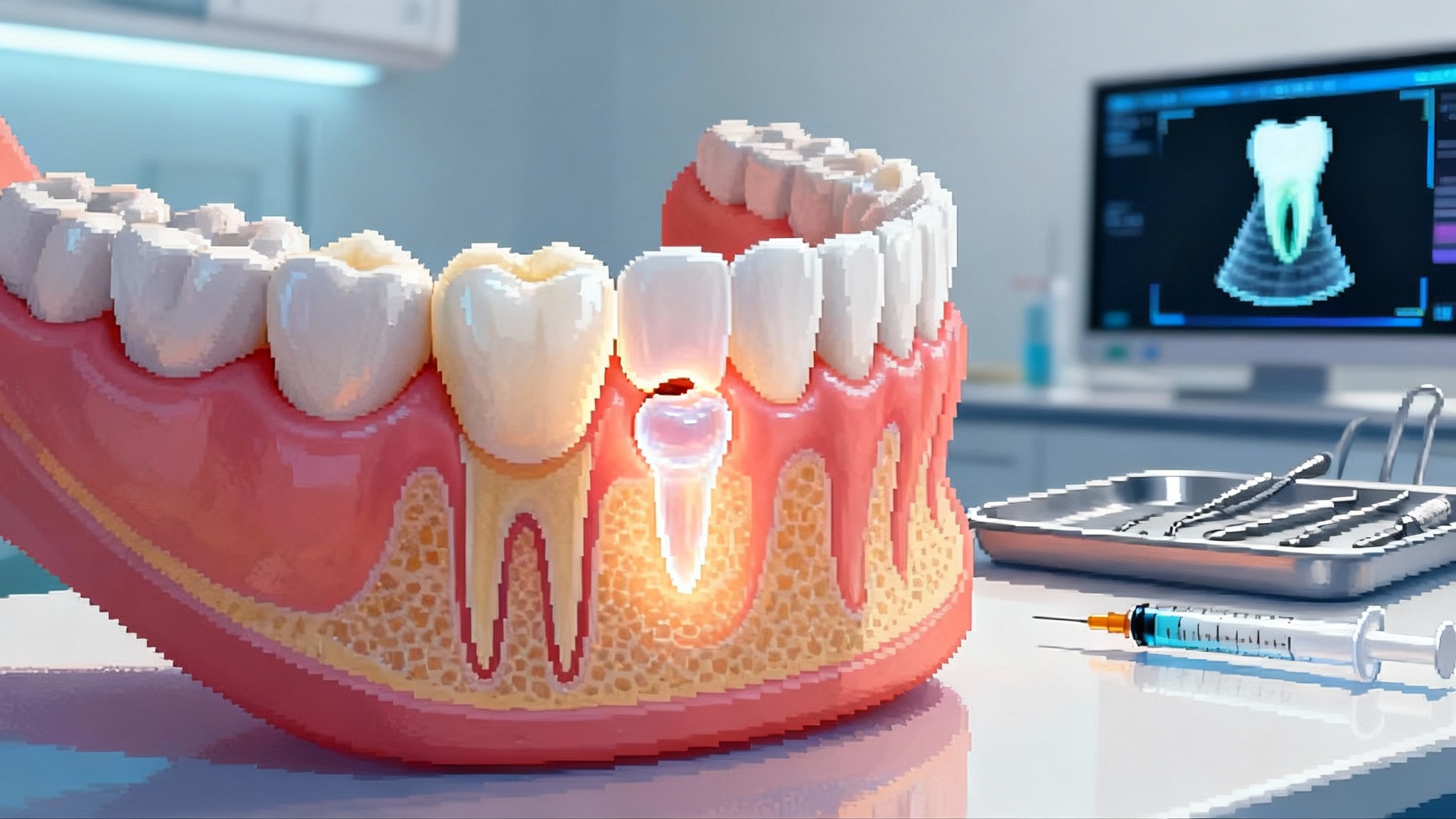
Teeth That Grow Back: TRG-035 Starts First Human Trials
Japan has moved tooth regeneration from lab promise to the clinic. Toregem BioPharma’s anti-USAG-1 antibody, TRG-035, has orphan status and is now in first-in-human testing, opening a new path beyond dentures and implants.
Pig Kidneys Go Clinical: The 2025 Xenotransplant Pivot
2025 marked the year xenotransplantation left the lab. The FDA cleared the first human trials of gene-edited pig kidneys, and one recipient lived nearly nine months with a single graft. Here is how trials, safety guardrails, and manufacturing are turning a moonshot into medicine.
CHIP Is the New LDL: 2025 Turns Clones Into Treatable Targets
Fresh 2025 evidence moves CHIP from risk marker to modifiable target. ESC data link low-dose colchicine to slower TET2 clone growth, an NIH-backed canakinumab trial is enrolling, CKD ties sharpen risk, and JAMA Cardiology cautions against aspirin for primary prevention.
Moderna's CMV Failure Sparks A Geroscience Pivot
On October 22, 2025, Moderna ended its congenital CMV vaccine program after a failed primary endpoint. The loss points to a bigger win: treating CMV as a modifiable driver of immunosenescence with vaccines, T cells, and antivirals built for older adults.