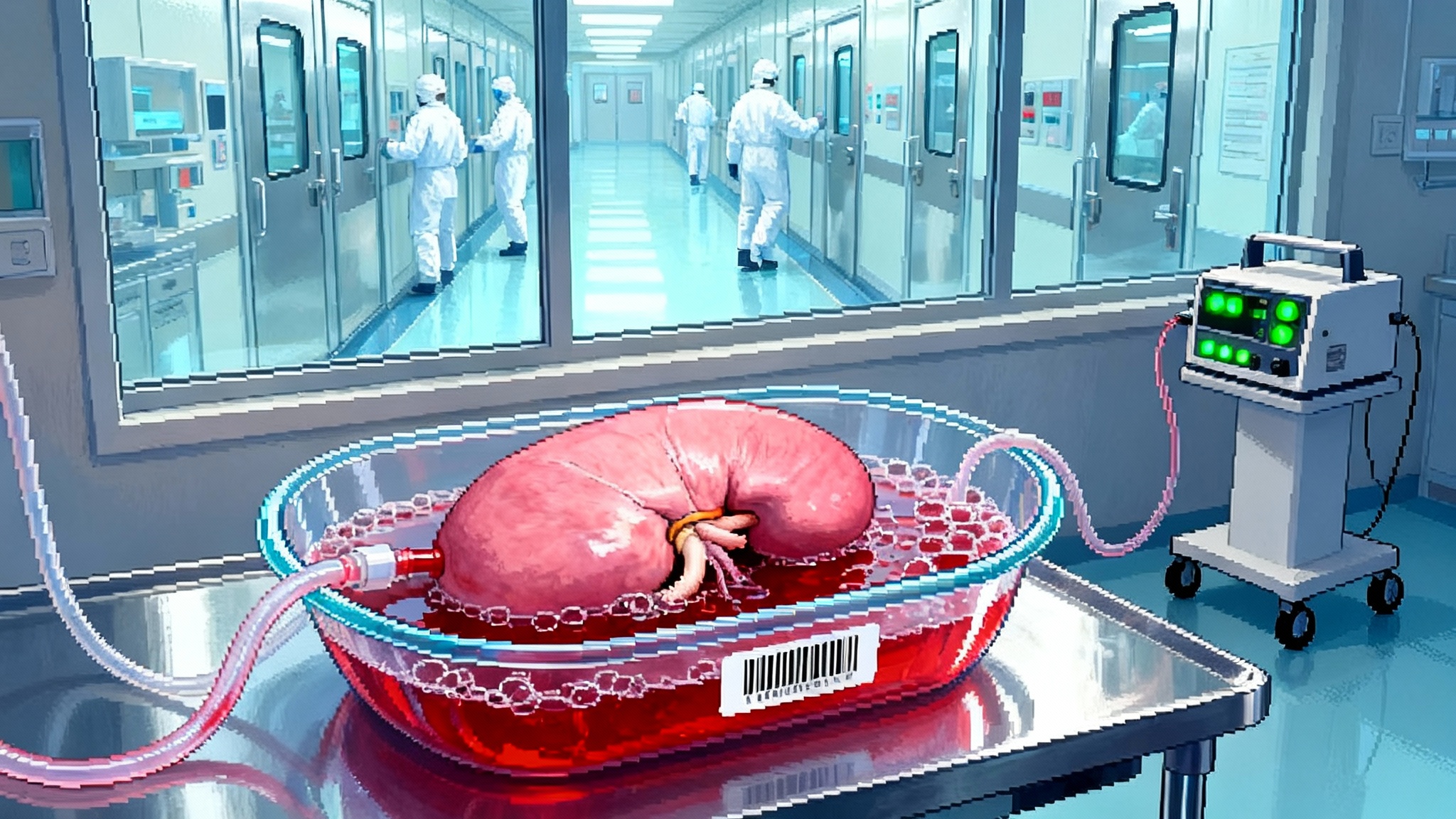
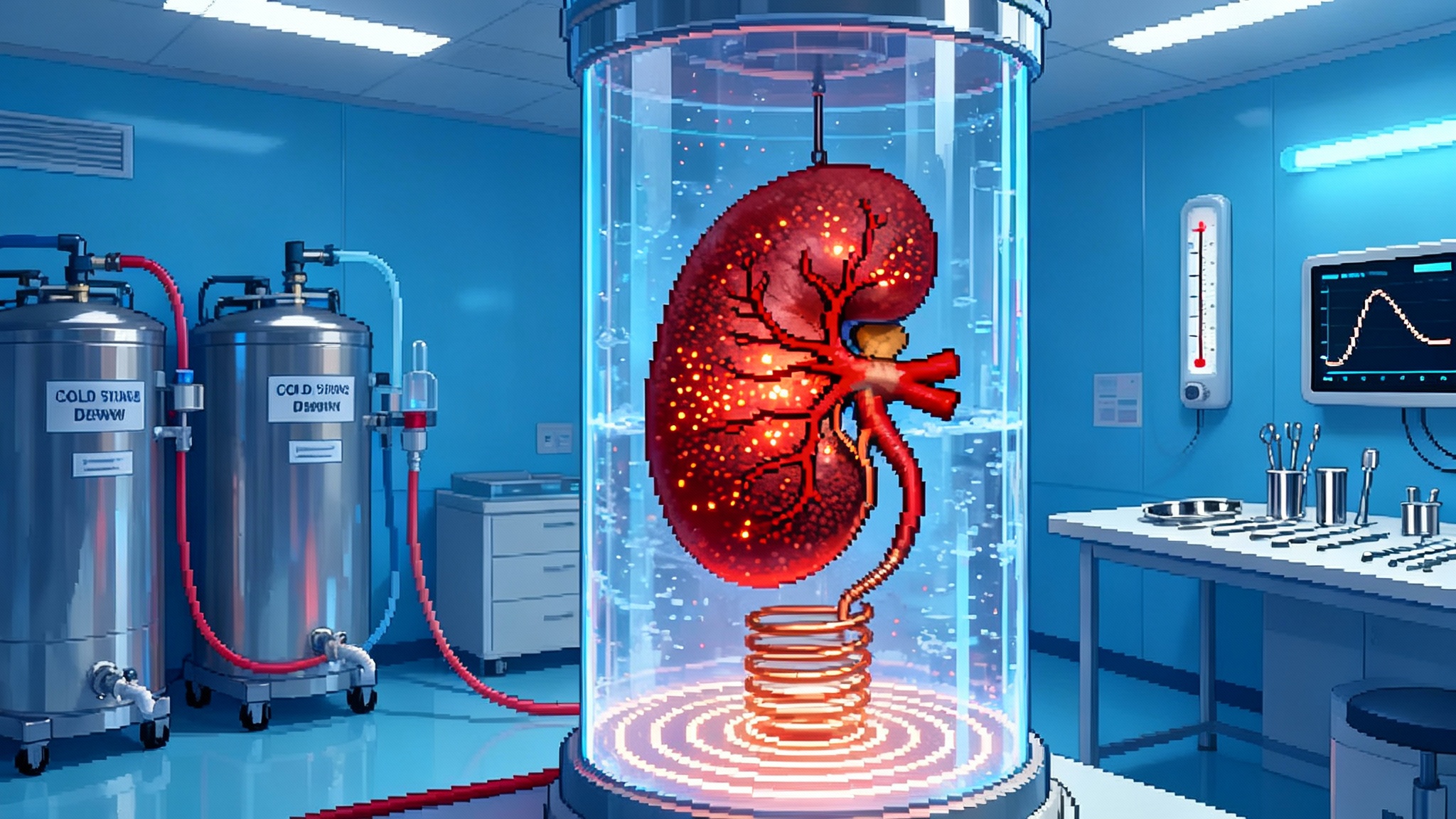
Nanowarming Brings Organ Banking to Human Scale in 2025
Two milestones in 2025 moved organ cryopreservation from elegant physics to practical engineering: a 10-day pig kidney transplant after cryostorage and liter-scale nanowarming that heats human-sized volumes uniformly. Here is what changed, why it matters, and what comes next.
The year organ banking snapped into focus
In 2025, a pair of milestones took organ banking from science-fair poster to engineering plan. In March, a team at Massachusetts General Hospital, working with the National Science Foundation’s ATP-Bio center, cryopreserved a pig kidney for 10 days, rewarmed it, and transplanted it back into a pig with restored function, a first for a large mammal and a dramatic leap in preservation time 10-day cryopreserved pig kidney transplant. Nine months later, a Nature Communications paper showed that vitrification and nanowarming could be pushed to liter-scale volumes, the size regime of human organs, while keeping heat uniform enough to avoid cracking or ice formation liter-scale vitrification and nanowarming.
For context on the clinical backdrop, see the related progress in xenotransplantation captured in our coverage of the xenotransplant pivot in 2025.
Together these results unlocked the last major bottleneck in organ cryopreservation: how to warm big, delicate organs fast and uniformly after they have been cooled into a glassy state. With rewarming demonstrated at meaningful scale, the field now looks less like speculative future and more like an engineering sprint.
Why rewarming was the hard part
Freezing damages living tissues because water expands as it turns to ice, rupturing cells and blood vessels. Vitrification avoids that by replacing much of the water with a cryoprotective cocktail and then cooling fast enough that the cocktail becomes a glass without forming crystals. Think of it as turning honey into a smooth toffee rather than a tray of jagged sugar shards.
The catch comes on the way back. If you rewarm slowly or unevenly, that glassy state can crystallize in a process called devitrification, and even small temperature gradients can crack tissue like a windshield shocked by boiling water. Small samples rewarm fine because heat can soak in quickly. Whole organs are different. A human kidney contains billions of cells, kilometers of vessels, and centimeters of thickness. Warming the surface with circulating fluid or a warm bath is like toasting a loaf of bread from the outside and hoping the middle does not remain frozen. It fails for the same reason bread burns before it thaws.
Nanowarming flips the geometry. Instead of pushing heat from the surface inward, it sprinkles heat sources throughout the organ’s vasculature. Iron-oxide nanoparticles are perfused through the organ when the cryoprotectant is loaded. Later, a radiofrequency coil excites those particles to produce heat volumetrically. The entire organ warms from the inside out and the outside in at once. Uniformity is the trick. The radiofrequency field penetrates centimeters deep, so all the nanoparticles heat together. When tuned correctly, a kidney can rise tens of degrees per minute without hotspots or cold cores.
What the liter-scale paper actually showed
The Nature Communications study tested three cryoprotectants in large volumes of half a liter to three liters. The authors confirmed that M22, a vitrification solution used in many organ studies, could be vitrified reliably at three liters and rewarmed at up to two liters using nanowarming, achieving average rates around ninety degrees Celsius per minute. That rate is not a flourish. It is above the critical threshold where devitrification tends to occur for these solutions and high enough to outpace the mechanical stresses that cause cracking.
They did more than warm beakers. The team vitrified porcine livers by perfusing them with cryoprotectant and demonstrated physical vitrification at organ scale. The first pass did not include a complete liver rewarm, which is harder than warming a bag of solution, but the heating hardware and control were validated at volumes that map onto human organs. They also showed that not all solutions behave equally at scale. VS55, another common cryoprotectant, failed at these volumes, while M22 worked up to the largest tested. That is actionable information for the next round of protocols.
To make this possible, the group built a high-power radiofrequency coil and mapped the field to keep heating even, then measured uniformity with thermometry and imaging. It is engineering meet biology, and it produced the first credible demonstration that liter-scale vitrification and rewarming can be done without ice and without cracks. The remaining steps look less like magic and more like systems integration.
The meaning of a 10-day kidney
The Massachusetts General Hospital experiment matters for a different reason. It closes the loop from storage to function in a large animal. The team removed a kidney from a pig, loaded cryoprotectant and nanoparticles via the vasculature, cooled it into a vitrified state, held it for 10 days, rewarmed it volumetrically, washed out the nanoparticles and cryoprotectant, and transplanted the organ. After surgery, the kidney perfused blood and produced urine.
A decade of organ preservation research has included beautiful physics and promising rodent data, but surgeons and patients need proof that a stored organ can be brought back to work. This is that proof in a large mammal. The absolute timeline matters too. Ten days is not just a convenience. It is the difference between racing a donated organ to the nearest suitable recipient and shipping it where it is most needed, matching it more precisely for immunology, and preparing a surgical team during daytime hours instead of midnight emergencies.
What rapid, uniform rewarming unlocks
Once rewarming is solved at human scale, everything up the chain changes.
- Match quality improves. With days rather than hours, transplant teams can perform more complete immunologic workups, reduce last-minute mismatches, and plan desensitization protocols. For the immunology backdrop, see how we frame treatable immune aging in 2025.
- Discard rates drop. Many organs are discarded for logistics rather than biology. A bank turns time from an adversary into an ally, allowing longer evaluation, repair on perfusion circuits, and consultation among centers.
- Surgery becomes elective. Teams can schedule during normal hours, assemble full staffing, and optimize care pathways. This lowers complications and cost and makes participation easier for centers with thin on-call coverage.
- Equity increases. Time banks allow organs to move across regions to the best recipient rather than the closest. Distance becomes logistics instead of destiny.
Kidneys will lead, because they tolerate manipulation and have established measures of function after transplant. Livers should follow, though they will likely lag by a couple of years because heating and perfusion uniformity are harder in a large, dense parenchyma. Hearts and lungs come later. The physics is similar, but the acceptable margin for injury is narrower and the washout protocols must be gentler.
From emergency to infrastructure
Think of organ banking as a shift from emergency medicine to infrastructure. Today’s system is a just-in-time network with perishable goods and panicked scheduling. Banking turns transplant into inventory management with quality control. That is not a slogan. It implies barcodes for organs, chain-of-custody, lot-tracked cryoprotectants, and validated rewarming coils with calibration certificates. The core capability is rapid, uniform rewarming. The rest is the logistics and quality system you would expect in a modern biomanufacturing line.
The 2026 to 2028 checklist
If 2025 was about demonstrating possibility, 2026 to 2028 must be about demonstrating safety, repeatability, and regulatory fit. Here is the shortlist that matters.
- Good Manufacturing Practice iron-oxide nanoparticles. The particles must be manufactured at scale with tight control of size distribution, surface chemistry, magnetic properties, and sterility. They need validated stability in cryoprotectant cocktails such as M22 across relevant temperatures and times, and a reliable washout protocol with acceptance criteria for residual iron in tissue.
- Device safety and classification. The high-power radiofrequency coil looks like a medical device, not a lab rig. Teams should prepare an Investigational Device Exemption package with data on electromagnetic exposure, heating uniformity, induced currents in surrounding hardware, and fail-safes. Expect shielding and interlocks to matter for health care worker safety.
- Combination product governance. Because nanowarming relies on both a device and a particle-plus-cryoprotectant solution, sponsors should expect combination product oversight. Coordinated plans that integrate Investigational New Drug toxicology for the nanoparticle and Investigational Device data for the coil will smooth review.
- Biocompatibility and clearance. Studies must quantify how much nanoparticle remains after washout, where it goes if any persists, and how quickly it clears. Look for magnetic resonance imaging and histology to confirm distribution and for serum markers to track clearance over weeks.
- Human discarded organ studies ex vivo. Before first-in-human transplant, the field should conduct multi-center studies using kidneys and livers declined for clinical use. Success means repeated vitrify-warm cycles with intact histology, preserved molecular markers, and normal perfusion on machine circuits for hours.
- Real-time thermometry and control. Fiber optic probes and magnetic field modeling will be standard. The goal is deterministic, not heroic, rewarming.
- Organ allocation modeling. Networks must simulate how banks change flows. Expect different rules for how long an organ can be held, how many offers can be made, and how to handle cross-regional prioritization.
A realistic cadence looks like this. In 2026, expect Good Manufacturing Practice nanoparticle lots, finalized washout protocols, and ex vivo studies on discarded human kidneys and livers under research protocols. In 2027, watch for survival studies in large animals with kidneys warmed after weeks of storage and for the first complete vitrify-rewarm-transplant cycle in large-animal livers with functional endpoints out to 90 days. In 2028, the first early-feasibility human studies should begin, likely focused on kidneys, with objectives centered on safety, delayed graft function rates, creatinine trajectories, and biopsy-proven absence of unusual injury.
Kidneys first, livers next by a one to three year lag, hearts and lungs after that. The exact calendar will move, but the order of operations will not.
The bottom line
In a single year, organ banking went from an elegant physics problem to a practical clinical plan. The 10-day cryopreserved pig kidney transplant showed that a stored organ can come back to work in a large mammal. The liter-scale nanowarming study showed that you can heat human-sized volumes fast and evenly enough to avoid the two classic failure modes, ice and cracks. What remains is disciplined engineering, manufacturing, and regulation. Do those well and transplant shifts from crisis response to scheduled care, with fewer deaths on the waitlist, more equitable access, and a new category of elective surgery. The technology to get there now fits in a single sentence. Warm fast. Warm evenly. Then the rest is logistics.