From Skin to Egg: IVG’s 2025 Breakthrough and What Comes Next
In September 2025, OHSU researchers reported human eggs made from skin cells and then fertilized in vitro. This review explains what was achieved, why it could extend reproductive longevity, the safety and regulatory gates ahead, and a realistic path to first pilots.
The moment IVG crossed a human threshold
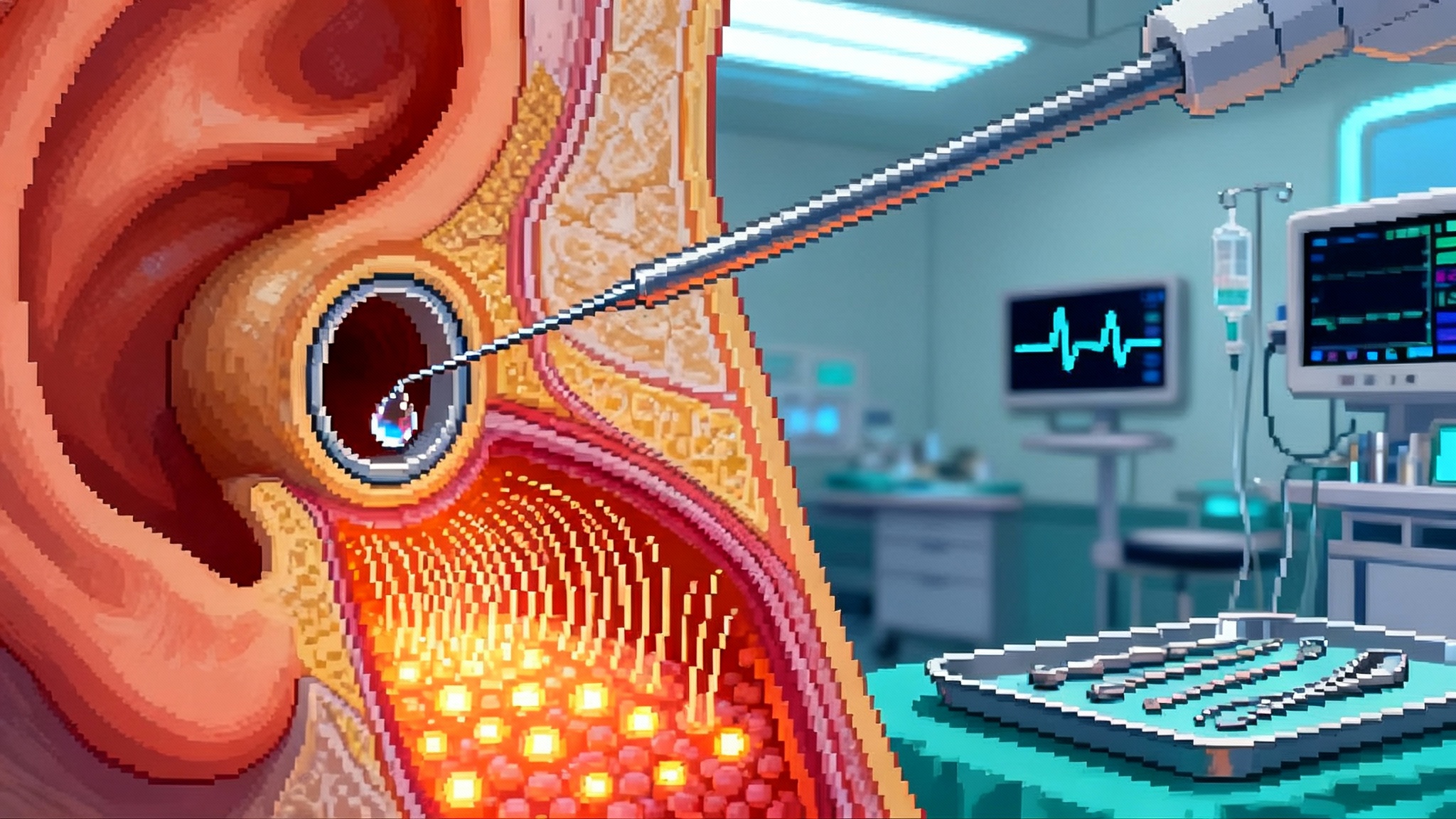
On September 30, 2025, a team at Oregon Health and Science University reported a striking result: they took human skin cells, turned them into eggs in the lab, then fertilized those eggs and grew early embryos to the blastocyst stage. The group coined a new term for the chromosome math that made it possible, mitomeiosis. It is a proof of concept with sharp limitations, but it marks the first time this full sequence has been shown with human material rather than mice. You can read the core announcement in the university’s release, which lays out the steps and numbers with useful clarity: OHSU researchers created fertilizable eggs.
What actually happened in the dish
The recipe is easier to follow if you picture a shell swap and a spring cleaning:
- Start with a donated human egg. Remove its nucleus, which holds 46 chromosomes.
- Take the nucleus from an ordinary skin cell of an intended parent. Place that nucleus inside the enucleated donor egg. This part is a classic somatic cell nuclear transfer, the basic move that produced Dolly the sheep.
- The egg’s cytoplasm then nudges the imported nucleus to jettison half its chromosomes. That halving is what the authors call mitomeiosis. If the halving is correct, the reconstructed egg ends up with 23 chromosomes, like a natural egg.
- Fertilize the reconstructed egg with sperm through standard in vitro fertilization.
By the numbers, the team created 82 reconstructed eggs, fertilized them, and saw roughly 9 percent reach the blastocyst stage at six days. Most embryos stalled earlier and showed chromosomal errors. None were cultured beyond six days. The authors were explicit: this is not ready for pregnancy, and likely will not be for years.
Mechanistically, the clever bit is the halving. In biology, cells divide by mitosis for growth and by meiosis for reproduction. Meiosis is not just division by two. It involves pairing maternal and paternal chromosome copies, swapping DNA segments at carefully placed crossovers, then separating the pairs with exquisite timing. The OHSU group found a way to induce a halving event from a somatic nucleus, but the pairing and crossover placement were often messy. That is why many embryos carried too many or too few chromosomes.
Why this matters for reproductive longevity
In humans, ovarian aging advances much faster than somatic aging. Egg quality declines in the mid to late thirties as cohesin proteins loosen their grip, crossovers get mispositioned, spindle checks grow leakier, and mitochondria lose performance. That is why aneuploidy in eggs rises steeply with age, and why many in vitro fertilization cycles fail even with excellent embryo grading.
In vitro gametogenesis offers an alternate route: generate new eggs from abundant somatic cells, then mature and fertilize them just as we do in IVF. If the gates to safety and fidelity can be crossed, IVG would partially decouple ovarian aging from chronological age. Two specific reasons make this plausible.
- Cytoplasmic reset: the cytoplasm in donor eggs is young and packed with mitochondria and factors that orchestrate chromosome segregation. That could sidestep the mitochondrial wear and spindle fragility that come with late maternal age.
- Epigenetic reboot: reprogramming and oocyte growth in the right environment can reset many age-associated epigenetic marks. It does not erase every DNA lesion a skin cell has picked up over decades, but it can reduce the functional age of the resulting egg.
There are caveats. Somatic cells accumulate point mutations and structural variants as we live. A rigorous pipeline would need to select starting cells with low mutational burden, sequence the resulting eggs or embryos deeply, and ensure that key imprinted regions are correctly set. For a broader look at how clonal genetics reshapes risk management, see our overview of CHIP as a treatable target.
The three gates between proof of concept and a clinic
A technology like this only earns real-world use by clearing specific, testable gates. Three loom largest.
1) Error-free meiosis in a lab context
What success looks like: high rates of correct homolog pairing and crossover placement, clean segregation, and a stable haploid set before fertilization. That means routine diploid embryos with normal karyotypes, not just occasional wins.
How to measure it: single-cell karyotyping of reconstructed eggs and early embryos, long-read sequencing to map crossovers and structural variants, and live imaging of spindle dynamics to prove that the halving step mimics the choreography of natural meiosis rather than a coin toss.
How to get there faster: rebuild more of the oocyte’s meiotic machinery in vitro. For example, supplement donor cytoplasm with purified or engineered cohesins and synaptonemal complex components, and tune kinase and phosphatase timing with small molecules so that chromosome pairing has time to complete before the reduction division. In mice, adjusting these clocks changed aneuploidy rates. Translating that to humans will take careful toxicology and staged ramp-ups.
2) Correct imprinting across development
Imprinting is the parent-of-origin marking system that controls a small but critical set of genes. Improper imprinting can cause syndromes like Beckwith-Wiedemann or Angelman. Because imprinting marks are set during gametogenesis and early embryo development, IVG must demonstrate that marks are erased, established, and maintained with high fidelity.
What success looks like: genome-wide imprinting profiles that match natural human eggs and embryos at the same stages, and that stay stable as embryos progress and after birth in animal models.
How to measure it: deep bisulfite sequencing at all known imprinted loci plus allele-specific expression assays across development. In nonhuman primates, follow offspring for years, then assay germline imprinting in their gametes to detect subtle multi-generational drift.
How to get there faster: mature reconstructed eggs in co-culture systems that mimic follicular signaling and metabolite levels rather than minimalist media. The maturing oocyte relies on nurse-like granulosa cells to lay down correct epigenetic marks. Rebuilding that microenvironment is a solvable engineering problem.
3) Off-target risks from the whole workflow
Even without gene editing, there are off-target risks. Somatic cell nuclear transfer can stress chromosomes, cause mis-segregation, or trigger chromothripsis. If any group uses CRISPR to fix a stubborn error, that adds the classic concerns about unintended edits. There is also a mitochondrial wrinkle. In this approach, mitochondrial DNA comes from the donor egg, not the skin cell donor. That could be beneficial for energy and error rates, but it introduces another genome that clinics must source, screen, and track.
What success looks like: whole-genome sequencing of embryos that shows low de novo mutation burden and no structural catastrophes, mitochondrial heteroplasmy under tight thresholds, and no unexpected off-target edits if editing is used.
How to measure it: trio sequencing with long-read platforms, single-cell multi-omics to catch rare clones with abnormalities, and tumorigenicity studies in nonhuman primates with long follow-up.
How to get there faster: build a standardized release spec that any IVG egg must meet before fertilization. Include karyotype, structural variant limits, aneuploidy risk models, imprinting panels, mitochondrial copy number and heteroplasmy caps, and a pass-fail for spindle integrity observed in live imaging. For parallels in high-spec genetic products reaching the clinic, see PCSK9 editing 2025 inflection.
2025 UK signals: what HFEA just told Parliament
The United Kingdom’s Human Fertilisation and Embryology Authority has been clear about two things. First, clinical use of in vitro derived gametes is currently prohibited by the law that governs fertility treatment. Second, the agency believes that IVG should be brought under explicit statutory regulation in time, with biologically dangerous uses never permitted, and that modernisation of the law is needed to keep pace with science. The regulator summarized that stance in a brief to government in 2025: HFEA recommendation on IVGs.
Two other UK trends matter. The HFEA has explored extending the 14 day research limit to allow more study of early development under strict oversight, and it has run a broader consultation on modernising the fertility law to address categories like in vitro derived gametes and stem cell based embryo models. If Parliament takes up these recommendations, the UK could become the first venue with a clear, staged pathway for IVG eggs to move from bench to clinic once science is ready. For a case study in how a regulatory sandbox can unlock first-in-human work responsibly, see the 2025 xenotransplant pivot.
The startup race, the academic bench, and realistic timelines
OHSU’s mitomeiosis paper puts a spotlight back on the academic front. In parallel, a quiet industrial race is underway.
- Conception Biosciences has pursued the classic route of reprogramming human cells to induced pluripotent stem cells, then directing those cells through a germ cell path, with the goal of building ovarian organoids that mature eggs. The company shares little publicly, but leaders in the field regularly place it among the likely frontrunners in the stem cell based track.
- Ivy Natal has built a small team around engineered cell systems and automation to accelerate germ cell specification and maturation. It is early, but its focus on machine learning guided protocols could matter once scaling becomes the bottleneck.
- Gameto is not making eggs from skin cells. Instead, it is upgrading today’s in vitro maturation with lab engineered ovarian support cells derived from induced pluripotent stem cells. Multiple clinics have launched studies of this approach. While it is an adjacent technology rather than IVG, a smoother, cheaper maturation step could plug directly into a future IVG workflow.
- In Japan, groups led by Katsuhiko Hayashi and Mitinori Saitou continue to set the pace in animal models, especially for the long, fiddly work of recapitulating meiosis and follicle dynamics in vitro.
What timing is credible if you are not selling a dream? The OHSU authors themselves caution that a decade of research may be needed before clinical trials are considered, and that is assuming permissive regulations. A reasonable base case is 8 to 12 years to first tightly controlled pilots, with the United Kingdom or Japan more likely than the United States because their regulators have clearer mechanisms to authorize novel reproductive work under strict guardrails. An aggressive but still reality anchored case is 5 to 7 years if two things happen fast: a sharp rise in the fraction of reconstructed eggs with normal karyotypes, and nonhuman primate data that demonstrate healthy live births with normal imprinting and early development.
An accelerationist but safe path to first pilots
Speed and safety are not enemies if you make safety the blocker at each gate. Here is a concrete playbook.
-
Publish standard go, no go benchmarks. The field should agree on thresholds before anyone fertilizes an IVG egg for transfer: for example, at least 70 percent of reconstructed eggs demonstrate correct haploidy by single-cell sequencing, at least 60 percent of resulting embryos are euploid by day 5, and imprinting panels match natural controls within predefined bounds.
-
Shift preclinical work into nonhuman primates now. Require two generations of healthy offspring with normal growth, behavior, fertility, and imprinting profiles before any human pilot. This is costly and slow, but it removes guesswork about developmental timing and physiology that mice cannot answer.
-
Build a regulatory sandbox with rolling audits. The HFEA is well positioned to license a small number of centers to conduct stepwise studies under public protocols, with data logged in near real time into a national registry. Outside the UK, a similar sandbox will be needed in whichever country reaches clarity first.
-
Make donor cytoplasm a first class product. Because reconstructed eggs carry mitochondria from donor cytoplasm, each batch should come with its own release tests: mitochondrial genome sequencing, copy number metrics, and tests for heteroplasmy hotspots. A central biobank can lower costs and standardize quality.
-
Harden the genomics. Bake whole-genome, long-read trio sequencing into the clinical workflow. Add single-cell assays to catch rare clones with structural variants. Run unbiased assays for off-target edits if any genome editing touches the process.
-
Pre-register ethics and consent. Use layered consent that explains mitochondrial inheritance, potential multi-generational follow-up, and what happens to data. Require third party counseling for same-sex parenthood and single parent scenarios to address social and legal implications in advance.
-
Audit manufacturing like a cell therapy. Treat reconstructed eggs as a living product with lot control, traceability, deviation reporting, and independent quality release. Borrow from playbooks used in cell and gene therapy manufacturing, where release testing is tied to clinical risk.
What to watch in the next 24 months
- A second lab repeats the mitomeiosis result and improves the karyotype distribution by altering the timing of the halving step.
- A primate lab reports reconstructed eggs that produce euploid embryos at day 5 with normal imprinting panels.
- A regulator issues a draft sandbox for IVG preclinical work, with explicit criteria tied to embryo research limits.
- Startups shift from narrative to data, releasing blinded sequencing benchmarks that can be compared to natural IVF embryos.
- Clinical in vitro maturation improves further and lowers the cost and discomfort of egg retrieval, which will be essential for any IVG pipeline that still needs donor cytoplasm in its early versions.
The bottom line
The OHSU study does not deliver babies, and it is nowhere near a clinical offering. What it delivers is a working human pathway that ties a skin cell to a fertilized egg through a novel halving step. That alone resets the map. For reproductive longevity, the promise is practical and specific: a path to parenthood for people without eggs, a way to lower the age penalty in egg quality, and an eventual decoupling of ovarian function from the calendar. The price of that promise is equally specific: error-free meiosis, correct imprinting, and a verified clean genome, each demonstrated at scale in animals before anyone considers a human pilot. If the field codifies those gates now, chooses regulators who can move with oversight rather than paralysis, and demands industrial grade quality control, the first careful pilots can happen on an ambitious timeline without gambling with health.