2025 Gene Therapy Restored Hearing, Presbycusis Is Next
In 2025, surgeons used AAV dual-vector gene therapy to restore hearing in children with OTOF deafness. The same delivery stack is now targeting age-related hearing loss; here is the pipeline, risks, and what to watch from 2026 to 2030.
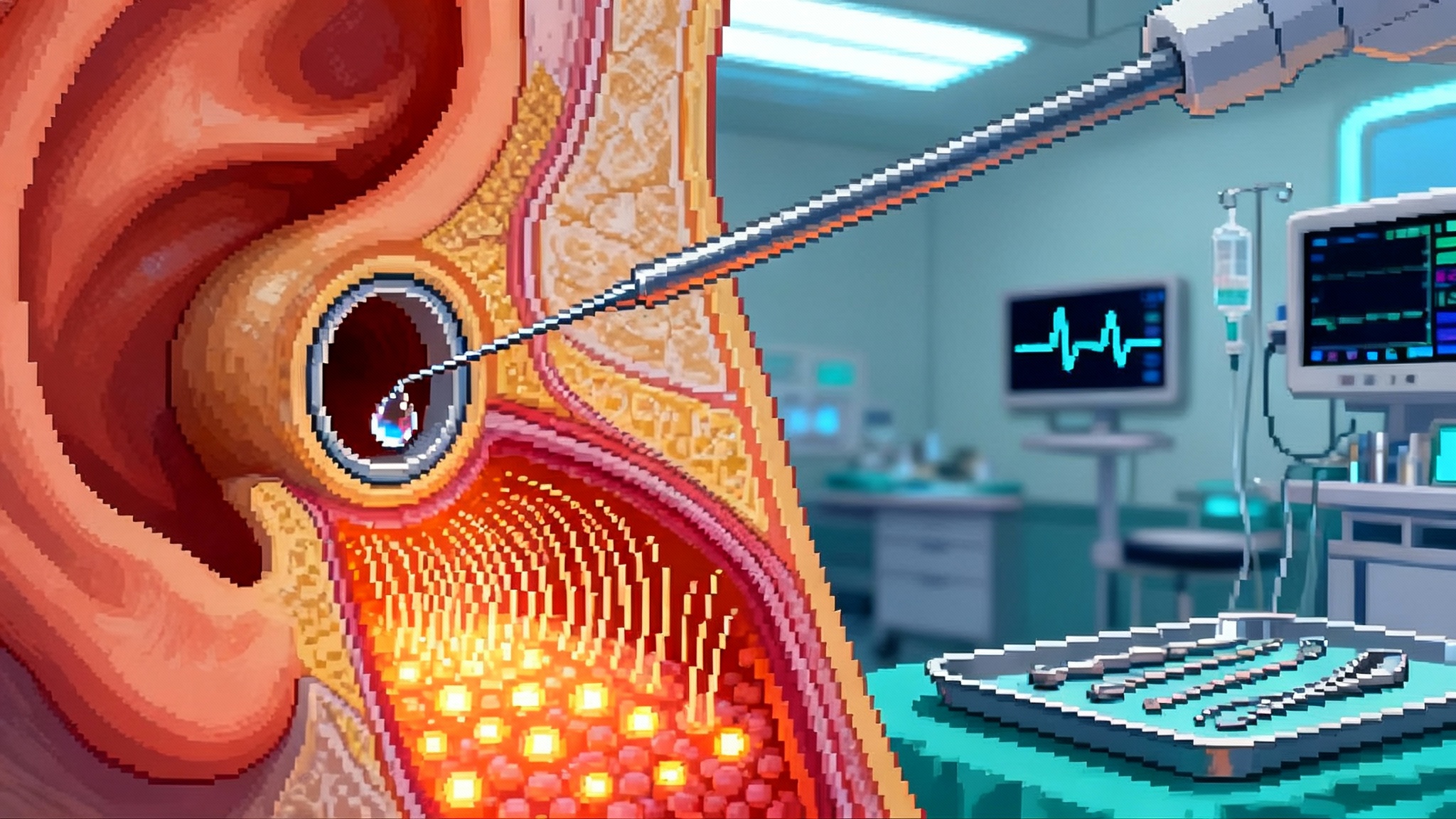
The moment hearing rebooted
In early 2025, a quiet revolution happened in operating rooms. Surgeons placed a microcatheter through the middle ear into the cochlea, infused two adeno-associated virus vectors carrying the halves of a large gene, and children who had never heard began responding to voices and music within weeks. In one program, 10 of 11 children with otoferlin-related deafness showed clinically meaningful gains, with some reaching near-normal thresholds, according to updated DB-OTO results. This is the kind of turning point that moves a field from proof of concept to platform.
The immediate story is thrilling on its own. But the larger story is what this delivery stack makes possible next. The same ingredients used to restore hearing in a rare pediatric condition are now being adapted for presbycusis, the age-related hearing loss that touches most families and flattens the richness of conversation, music, and daily life.
What changed inside the cochlea
Think of the inner ear as a piano tucked deep in bone. Hair cells are the keys, the auditory nerve is the string section, and neurotransmitter release is the hammer action that turns vibration into electrical signals. In otoferlin deficiency, the hammer is missing. Dual-vector adeno-associated virus delivery gets around the size limit of the virus by splitting a big gene into two parts that rejoin in the target cell. Surgeons guide a small cannula into the cochlea, gently infuse the vectors, and the rebuilt otoferlin protein restores the hammer action at inner hair cells. The result is not louder sound, it is sound that finally makes it from key to string.
Three elements proved decisive in 2025:
- A cell-selective promoter to confine expression to inner hair cells, which reduces off-target risk.
- Dual vectors that reconstitute the large OTOF coding sequence, which overcomes the packaging limit of adeno-associated virus.
- A surgical technique borrowed from cochlear implantation and refined for fluid-calm delivery, which protects the delicate structures that do not regenerate on their own.
From rare deafness to common aging loss
Presbycusis is not one disease. It is a cluster of failures accumulating over decades. Two stand out as near-term gene therapy targets.
- Cochlear synaptopathy. Repeated noise, vascular changes, and aging prune the synapses that connect inner hair cells to spiral ganglion neurons. This is why speech in a noisy restaurant becomes hard even when the audiogram looks normal. Restoring or stabilizing these synapses is a practical gene target because the cells remain, but the connections fade.
- Hair-cell attrition. Outer hair cells, the cochlea’s built-in amplifiers, die off with age and toxin exposure. Inner hair cells can persist but transmit poorly when support systems fail. Here the frontier is reprogramming of supporting cells into hair-cell-like cells or delivering protective factors that slow the slide.
OTOF programs proved that inner ear gene delivery can be precise, tolerated, and lead to meaningful hearing. The next wave reuses the same tools to deliver neurotrophins and protective genes, and to modulate ion channels or gap-junction proteins that maintain homeostasis. Crucially, several groups are already moving common genetic contributors, like GJB2 variants that can drive early adult decline, toward the clinic.
The delivery stack that generalizes
If the field sounds like software, that is on purpose. We now have a stack:
- Capsid selection. An adeno-associated virus shell with high inner hair cell transduction and low off-target spread.
- Regulatory cassette. Promoters and enhancers tuned for either inner hair cells, outer hair cells, or supporting cells, with expression levels that are strong enough to work but not so high that they cause toxicity.
- Dual-vector assembly. For large payloads, overlapping or trans-splicing designs rejoin code inside the cell. For smaller payloads, a single vector is simpler and leaves headroom for regulatory sequences.
- Access device. Transcanal, round-window, or soft cochleostomy approaches, paired with micro-flow control, limit turbulence and shear.
Once this stack is validated and standardized, changing the “app” from otoferlin to a synapse-protecting protein or a hair-cell regeneration factor becomes a realistic development plan rather than a moonshot. The same platform thinking is emerging in one-shot OA gene therapy for joints, where delivery and durability define the moat as much as the payload.
Technical risks that still matter
The inner ear is small, exquisitely sensitive, and partially walled off from the immune system. That makes safety solvable but non-trivial.
- Dose and fluid dynamics. Too little vector, no effect. Too much, and pressure spikes or off-target transduction can damage residual function. Programs are converging on dose ranges measured by vector genomes per ear, delivered over minutes with real-time monitoring. Expect manufacturers to publish target flow rates and pressures the way stent makers publish expansion curves.
- Immune priming and bilateral timing. Adeno-associated virus exposure can trigger neutralizing antibodies. If the second ear is treated later, titers might rise and blunt efficacy. Trials have tested one-ear first, then bilateral dosing at the same session or within a short window before the immune system fully responds. For presbycusis in adults, many candidates will already have adeno-associated virus antibodies from natural exposure. Developers are exploring immune modulation and engineered capsids that dodge common antibodies, but this will shape patient selection and re-treatment rules.
- Re-dosing. If gene expression wanes or disease progresses, reinfusion may be desirable. Classic adeno-associated virus re-dosing is difficult. Solutions include using a different capsid, transient immune suppression, or shifting to non-viral delivery. Plans that count on lifelong effect from a single dose will need credible durability data to convince payers and clinicians.
- Biodistribution and off-target expression. Even tiny misexpression in the vestibular system can cause dizziness. Hair-cell-specific promoters and lower-leakage constructs are becoming standard. Preclinical packages that include behavioral vestibular testing and high-resolution mapping of expression will be important signals of quality.
The 2026 to 2030 pipeline, decoded
-
- First regulatory filings for pediatric OTOF programs are plausible in the United States and Europe if durability and safety hold. A European GJB2 program is slated to enter first-in-human testing in early 2026. Watch for initial adult cohorts when the mutation causes progressive loss rather than congenital silence, since that is a bridge toward presbycusis.
-
- OTOF approvals in at least one major market are credible. More sites adopt standardized inner ear delivery devices, and bilateral dosing protocols become routine. Early clinical signals from synaptopathy programs that deliver neurotrophins to stabilize or re-grow ribbon synapses may appear in small, well-phenotyped adult populations who struggle with speech-in-noise despite relatively preserved thresholds.
-
- Combination strategies emerge. One ear receives a synapse-supporting payload while the other ear receives a hair-cell survival payload, or a single cassette delivers both under different promoters. Expect mid-stage trials in adults with age-related hearing loss defined by speech-in-noise deficits and reduced electrocochleography amplitudes, not just by standard audiograms.
-
- First pivotal studies for a presbycusis gene therapy begin if safety is clean and effect sizes are clinically meaningful. These trials will likely anchor on functional endpoints that matter to daily life, such as multi-talker speech understanding and localization, with digital speech tests collected in homes.
-
- Centers of excellence in otology perform gene delivery alongside cochlear implant surgery and complex stapedotomies. Medicare and commercial insurers pilot outcomes-based contracts that pay over time if hearing gains persist. Genetic screening panels become standard for adults with early decline to triage candidates for gene augmentation. Surgical adoption will likely mirror other healthspan-frontier procedures such as pig kidney trials and healthspan, where specialized centers prove safety and repeatability first.
Companies to watch include Regeneron, which integrated Decibel’s platform, Eli Lilly’s Akouos unit, Sensorion with Institut Pasteur on GJB2 and OTOF, and academic groups that demonstrated bilateral gene delivery in children. The shared theme is not any single gene. It is the maturing craft of safely getting therapeutic code into the cochlea and making it stick.
Regulatory and pricing outlook
Regulators have already given pediatric OTOF programs rare pediatric disease and orphan designations, which can accelerate review. For adult presbycusis, expect a more conservative posture: larger safety databases, robust functional endpoints, and long follow-up. Early use will concentrate in specialized centers with intraoperative monitoring and standardized delivery.
On pricing, history is instructive. One-time gene therapies launch between 850,000 dollars and 3.5 million dollars depending on disease prevalence, severity, and durability claims. OTOF is ultra-rare and surgical, so list prices may land in the upper tier with outcomes-based rebates. Presbycusis cannot follow that script at population scale. To cross from rare disease to mainstream aging care, developers will need to:
- Reduce per-dose cost through higher-yield manufacturing and smaller doses enabled by better capsids.
- Offer annuity or pay-over-time models that fit Medicare and employer plans, with refunds tied to durable speech-in-noise gains at two and three years.
- Publish five-year durability curves and real-world safety data so payers can model budget impact credibly.
If a gene therapy can delay or avoid a cochlear implant in even a fraction of older adults, the health economics become compelling. Implants require surgery, hardware, programming, and replacement cycles, and although they are transformative for many, they do not replicate natural acoustic hearing. Durable preservation of biological hearing would change lifetime costs and outcomes.
Trials to keep on your short list
- CHORD, the DB-OTO program in infants through adolescents with OTOF deficiency, the clearest precedent for inner ear gene delivery at scale.
- AK-OTOF-101, Eli Lilly’s Akouos program, which pairs a purpose-built delivery device with a dual-vector cassette. Device performance and dose selection will inform presbycusis delivery.
- Audiogene, Sensorion’s OTOF program and its companion natural history study. The same group’s GJB2 program aims at progressive loss that includes early adult decline, a key stepping stone toward age-related loss.
- Academic bilateral OTOF trials in China in partnership with global investigators, important for understanding immune timing and surgical choreography when both ears are treated.
Signals that presbycusis gene therapies are moving from rare to mainstream include: adult cohorts entering gene trials based on speech-in-noise deficits; regulatory acceptance of functional composite endpoints, not just pure-tone thresholds; publication of standardized surgical and fluid-delivery parameters; payer pilots with outcomes contracts; and the appearance of genetic testing pathways for adults with early decline, particularly for common contributors like GJB2 variants.
Hearing and healthspan
Hearing is not only about sound. It is cognitive workload, social connection, movement safety, and brain health. In a large randomized trial, a multiyear hearing intervention slowed cognitive decline for older adults at higher dementia risk, as summarized in the NIH summary of ACHIEVE. The mechanism is simple and profound. When people hear clearly, they engage more, think less about deciphering syllables, stabilize gait through better environmental cues, and maintain conversations that keep networks and mood intact. Preservation thinking is showing up across aging medicine, from Alzheimer’s blood tests in 2025 to prevention-first gene therapies.
Gene therapy slots into that logic as a prevention and preservation tool. Imagine a 62-year-old with early synaptopathy who struggles at work meetings. Today, the options are amplification, training strategies, and environmental tweaks. Within five years, the conversation could include a one-time synapse-supporting cochlear infusion at a specialty center, followed by objective speech performance monitoring on a phone. If the gains persist, insurance continues to pay. If they fade early, the contract rebates automatically. That is a practical bridge from rare pediatric programs to adult care.
What to do now if you are a stakeholder
- Clinicians. Begin documenting speech-in-noise performance and tinnitus burden for older adults, not just audiogram thresholds. Build referral relationships with centers testing inner ear delivery so appropriate patients can be triaged quickly when adult cohorts open.
- Employers and payers. Pilot hearing-health benefits that include baseline speech-in-noise testing at age 50, coverage for high-quality amplification, and clear pathways to gene therapy trials. These datasets will help you model budget impact and outcomes contracts later.
- Policymakers. Encourage newborn and adult hearing genetic panels in integrated health systems with strict privacy rules. Fund multi-site registries that follow outcomes of inner ear gene delivery for at least five years.
- Builders and investors. Fund delivery hardware and manufacturing, not just payload discovery. A safer, more repeatable surgical experience is a competitive moat that also reduces cost of goods.
The takeaway
Hearing restoration in 2025 was not a one-off miracle. It was the first production run of an inner ear gene delivery system that can be retargeted. The next chapter is presbycusis. The science is lining up around synapses and hair cells, the surgical craft is maturing, and early regulatory pathways are clear enough to navigate. Watch for adult cohorts, standardized devices, and outcomes-based pricing to mark the transition from ultra-rare rescue to mainstream preservation. When we keep people hearing, we keep them thinking, moving, and connected. That is not only good medicine. It is added life, lived out loud.