One-shot OA gene therapy could bend the healthspan curve
Peer-reviewed 2025 data show a single AAV knee injection can drive year-long IL‑1Ra expression, while Pacira’s PCRX‑201 posts three-year follow-up and advances to Phase 2. Durable, local gene delivery may finally modify osteoarthritis.
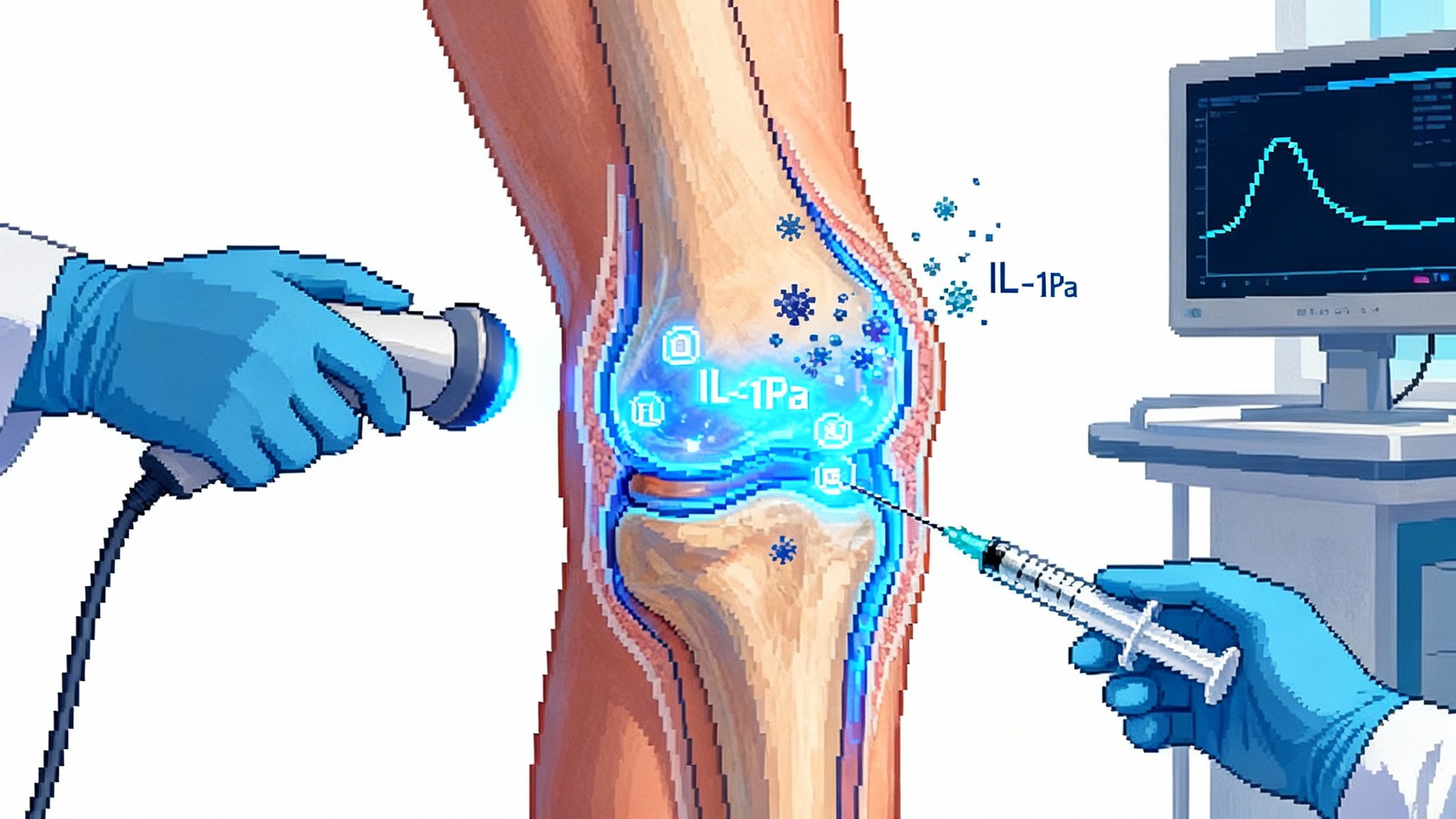
The breakthrough that puts movement back at the center of aging
In October 2025, the osteoarthritis field crossed a psychological and scientific threshold. A peer-reviewed phase 1 study reported that a single intra-articular adeno-associated virus injection into the knee could drive sustained production of interleukin‑1 receptor antagonist for a full year, with no serious vector-related adverse events and measurable gains in patient-reported pain and function. The paper, published earlier in 2025 and widely discussed by clinicians this fall, showed elevated synovial fluid levels of the therapeutic protein and a humoral but not cell-mediated immune response in the joint, an immunology profile that matters for repeat-access to care models and long-term safety. The idea is deceptively simple: turn the knee into a small, contained protein factory that raises the anti-inflammatory thermostat only when the joint flares. Similar one-shot durability is reshaping lipids in Lp(a) single-shot programs. It is the first clear sign that one-shot gene therapy could alter the trajectory of osteoarthritis rather than just dull it. See the study details in a peer-reviewed journal summary of the phase 1 trial in Science Translational Medicine, which documented year-long intra-articular expression after a single dose and minimal systemic exposure. Phase 1 knee IL‑1Ra expression.
On a parallel track, Pacira BioSciences delivered three-year follow-up from its high-capacity adenovirus program, PCRX‑201, after a single knee injection in a phase 1 cohort, and it has already dosed patients in an active randomized phase 2 trial. The long follow-up matters because it supports durability and informs regulators on what a realistic benefit window might look like in the real world, where arthritis ebbs and flows with activity, weight, and weather. Pacira’s three-year update also strengthens the case that a local, one-and-done approach can meaningfully outlast steroid or hyaluronic acid injections, which typically fade within months. For the three-year dataset and the program’s current status, see Pacira’s 2025 disclosure. Pacira’s three-year PCRX‑201 data.
Taken together, these two threads reshape a basic question that patients and surgeons have asked for decades. Can we slow the slide from occasional soreness to bone-on-bone? The new answer is not simply hope. It is a concrete path built around durable, local gene delivery that could delay joint replacements, extend independence in later life, and bend the healthspan curve toward more mobility.
Why IL‑1 and why the knee compartment
Osteoarthritis is not only about worn cartilage. It is a chronic inflammatory process seeded by mechanical stress, micro-injury, and age-related shifts in the synovium and subchondral bone. Interleukin‑1 acts like a smoke alarm that never fully turns off, driving pain and cartilage catabolism. Interleukin‑1 receptor antagonist is the body’s native counterweight that quiets the alarm. Injectable anakinra, the approved recombinant protein, has shown only transient benefit in the joint. It clears quickly, so any relief is measured in days to weeks. Gene delivery solves the pharmacokinetic problem by producing the same protein where it is needed and for as long as the transgene keeps working.
The knee joint is a rare gift to translational medicine. It is a large, accessible, mostly closed compartment. A single ultrasound-guided injection reaches the synovial lining that drives inflammation. Unlike systemic gene therapy that must navigate the liver and the bloodstream, intra-articular dosing keeps exposure local. In the AAV study, less than one percent of vector genomes were detected transiently in circulation and cleared within a week. That containment is not only a safety feature. It is the economic rationale for a therapy that may be priced and delivered more like an in-office procedure than a hospital-based infusion.
Two vectors, two philosophies of engineering
Beneath the headline results sit two distinct tech stacks that reflect different bets on immunology, durability, and manufacturability.
-
AAV for compact, stealthy delivery. The AAV program uses a self-complementary AAV capsid engineered for joint tropism and a compact expression cassette encoding interleukin‑1 receptor antagonist. The cartridge is small because AAV’s cargo space is limited. The upside is a well-studied vector biology, broad manufacturing know-how, and an immunology profile that is often quieter in tissues than older adenoviral platforms. The phase 1 knee study documented humoral antibody formation against the capsid but no cell-mediated response, with sustained intra-articular protein expression over twelve months. As with cardiovascular editing, see PCSK9 base editing’s one-and-done leap.
-
High-capacity adenovirus for big payloads and an inflammation-responsive switch. Pacira’s PCRX‑201 uses a helper-dependent, or high-capacity, adenoviral vector that carries more DNA. That headroom enables an inflammation-inducible promoter. In plain language, the cassette behaves like a dimmer switch that turns up interleukin‑1 receptor antagonist during a flare and turns it down as the joint quiets. The platform also allows steroid preconditioning to tame innate responses at the moment of injection. The company’s open-label phase 1 experience suggests multi-year clinical benefit after a single dose. Its ongoing phase 2 is designed to quantify safety, function, and pain over the first year while tracking biomarkers of structure.
The convergence is striking. Both approaches aim to deliver the same native protein locally. Both are exploring steroid pre-treatment to optimize gene transfer and tolerability. Both are aiming at one-and-done dosing in the clinic. Yet they may not be interchangeable. AAV’s capsid-specific immunity can complicate repeat dosing in the same joint. High-capacity adenovirus may provoke stronger innate responses but avoids integration and offers larger engineering canvases for future payloads such as multi-gene cassettes.
Safety and immunology, explained simply
Think of the joint as a neighborhood. AAV is the quiet contractor who parks a small van, renovates the house, and leaves behind a gadget that keeps the thermostat steady. High-capacity adenovirus is a larger crew with a box truck who installs a smart thermostat that reacts to the weather. Both get the job done if the neighbors accept the work.
Here is what safety looks like so far and why it matters.
- Local reactions are common and manageable. Effusions, temporary swelling, and transient pain spikes have been observed after dosing. These events have resolved with conservative care in early trials.
- Systemic exposure is low. In the AAV phase 1 study, vector genomes in blood were minimal and rapidly cleared, aligning with the compartment concept and lowering the risk of off-target effects.
- Antibody formation is expected. Neutralizing antibodies to the AAV capsid rose in serum and synovial fluid. That is typical and may limit the feasibility of repeat dosing with the same capsid. It argues for careful choice of dose, steroid preconditioning strategies, or the use of alternative capsids in future cohorts.
- No integration, low theoretical insertional risk. Both AAV and high-capacity adenovirus predominantly remain episomal in joint cells. That reduces the risk of insertional mutagenesis compared with integrating vectors.
- Inducible control could widen the safety margin. PCRX‑201’s inflammation-responsive promoter is not a gimmick. By throttling protein production upward only when cytokine signals are present, it reduces the chances of overshooting physiologic levels during quiet periods.
The surveillance burden will not be trivial. Expect five-year follow-up per subject in registrational trials, a focus on vector shedding, joint imaging, and a special eye on immunogenicity for those who later undergo arthroplasty.
The regulatory playbook is taking shape
Regulators now have real human biomarker data and multi-year follow-up to work with. Two U.S. Food and Drug Administration designations are particularly important.
-
Regenerative Medicine Advanced Therapy for both modalities. PCRX‑201 received RMAT designation in 2024, and this summer GNSC‑001, an AAV interleukin‑1 receptor antagonist program, also received RMAT. RMAT signals that the Food and Drug Administration sees the potential for substantial improvement over existing options and offers intensive guidance on endpoints, accelerated approval routes, and post-approval evidence plans. For broader context on gene therapy’s path, see Klotho gene therapy race to trials.
-
Advanced Therapy Medicinal Product in Europe for PCRX‑201. That flags intent to coordinate with European regulators on endpoints that could include imaging and biochemical markers tied to structural change.
What might an approvable endpoint look like in osteoarthritis, a disease notorious for slow structural change? A practical path is a co-primary framework that pairs function and pain with a structural readout that can move inside a year. Candidates include quantitative magnetic resonance imaging of cartilage thickness or T2 mapping, bone marrow lesion volume, or synovitis on contrast-enhanced sequences, all supported by targeted biomarker panels of cartilage breakdown and repair. Regulators can then require longer-term structural follow-up post-approval to confirm disease modification.
The payer math favors a site-of-care model
Durable local gene therapy is poised to live in the same neighborhood as today’s steroid and hyaluronic acid injections, not in the high-cost gene therapy centers that serve inherited diseases. That matters for access.
- Setting and staffing. Ultrasound-guided intra-articular injections are standard in sports medicine and rheumatology clinics. Training in vector handling and adverse event monitoring can be added without new buildings.
- Payment mechanics. A separate product code with per-joint billing is likely. Because the administration is familiar, reimbursement can follow the established office-visit plus procedure pattern. Expect outcome-based add-ons in payer pilots that tie continued coverage to maintained function at 6 to 12 months.
- Budget impact framed by surgeries avoided. The cleanest argument is delay of total knee replacement in higher-risk patients. Health plans will model time-to-arthroplasty and fracture risk from deconditioning to estimate return on investment. If one injection can reliably delay surgery by two to three years in the right segment, the therapy will slot into value-based musculoskeletal bundles quickly.
- Patient selection and step edits. Coverage will likely require radiographic confirmation, failure of oral nonsteroidal anti-inflammatory drugs and at least one intra-articular steroid, and body mass index counseling. That is a fair trade if the product preserves access for those who can benefit most.
A near-term timeline from 2025 to 2028
This is what a realistic arc looks like if programs hit their marks.
-
2025
- Peer-reviewed human AAV data on sustained interleukin‑1 receptor antagonist expression is published and discussed widely among clinicians.
- Pacira presents three-year follow-up from phase 1 and continues dosing in its randomized phase 2 trial. Manufacturing scale-up and stability data advance in the background.
- AAV developers complete multicenter phase 1b with biomarker and safety readouts, informing dose, steroid preconditioning, and inclusion criteria for the next study.
-
2026
- Pacira reports initial topline from the first part of its phase 2 trial late in the year. The readout spotlights safety, pain, function, and early structural markers. Design of the confirmatory study is finalized with Food and Drug Administration input under RMAT.
- AAV programs kick off phase 2 dose-ranging with a co-primary endpoint strategy that matches function with a structural biomarker panel.
-
2027
- If signals hold, Pacira begins a pivotal or registrational study with imaging plus patient-reported outcomes and long-term follow-up. Health systems launch real-world readiness pilots that integrate ultrasound workflows, steroid preconditioning protocols, and adverse event monitoring dashboards.
- AAV programs expand into multi-site phase 2b and test alternative capsids or dosing regimens to address neutralizing antibodies and repeat dosing questions.
-
2028
- Earliest window for an accelerated submission by a local gene therapy program if phase 2 shows convincing function and structural change under RMAT guidance and a confirmatory plan is locked in. Parallel payer pilots evaluate time-to-arthroplasty and physical function maintenance at the health-plan level.
This sequence is ambitious, but it is grounded in what sponsors have publicly guided and in what regulators have already signaled through designations.
Real-world deployment scenarios clinicians can plan for now
-
One-and-done for the active 60-year-old with moderate osteoarthritis. A single knee injection, a short steroid preconditioning protocol, and remote monitoring of pain and function scores. The goal is to extend the hiking and pickleball years while postponing surgery.
-
A bridge for the 70-year-old with severe osteoarthritis and comorbidities. The injection buys time to optimize weight, diabetes control, and physical therapy before any surgical decision.
-
A specialty program inside a health system’s musculoskeletal service line. Sports medicine and rheumatology run the clinic days. Orthopedics leads the registry and imaging review board. Payers fund outcome-based contracts pegged to six and twelve-month function thresholds.
-
A second-joint strategy. Because neutralizing antibodies may limit repeat dosing with the same AAV capsid, clinics prioritize the more symptomatic knee first and consider contralateral treatment on a separate timeline or with a different vector.
What to watch in the data over the next 24 months
- Structure, not just symptoms. Look for magnetic resonance imaging evidence of cartilage preservation, reduced synovitis, and smaller bone marrow lesions. Consistent structural benefits will separate disease modification from long-lasting analgesia.
- Immunology tuned by protocol. Programs are testing oral or local steroid preconditioning and timing of synovial fluid sampling. Expect higher and more durable expression when immune conditioning is used thoughtfully.
- Durability at scale. Three-year benefits in small cohorts are encouraging. The question is how much durability survives when hundreds of patients are treated across many sites.
- Arthroplasty outcomes. Surgeons will track wound healing, infection rates, and implant performance in previously dosed joints. Clean safety signals here will build confidence.
The bigger picture for longevity and independence
Mobility is the daily currency of healthspan. It is what lets older adults shop, climb stairs, garden, and see friends. If local gene therapy can reliably keep knees quieter for years, we will not just trim pain scores. We will change gait speed, fall risk, and hospitalizations downstream. That is how a joint-level therapy bends a life-level curve.
There will be debates about price, step therapy, and the exact definition of disease modification. That is healthy. The proof will come from registries that track time-to-arthroplasty and function in real clinics.
The most important near-term actions are concrete.
- Health systems should stand up small OA gene therapy pilots inside musculoskeletal service lines, with standardized ultrasound guidance, safety checklists, and patient-reported outcome collection at one, three, six, and twelve months.
- Payers should launch outcome-based contracts tied to function thresholds and time-to-arthroplasty, with special access programs for high-risk patients who could gain the most independence from delaying surgery.
- Sponsors should pre-register imaging and biomarker endpoints that read inside a year, publish their steroid preconditioning playbooks, and commit to five-year follow-up registries that are open to independent analysis.
- Clinicians should talk with patients now about realistic goals. Relief in weeks, function in months, and, if we are lucky, structural stability across years. Set expectations, measure what matters, and refuse to chase short-term wins at the expense of long-term joint health.
The bottom line
Osteoarthritis has long been a story of waiting: wait for the next injection, wait for the x-ray to look worse, wait for the right time to replace the joint. The 2025 data suggest a different arc. One carefully engineered shot can turn the knee into a self-regulating, anti-inflammatory factory for a year or more, and in some cases for several years. If phase 2 and pivotal trials confirm what early studies show, durable local gene therapy will not only delay many joint replacements. It will also let more people finish their own errands, on their own feet, later in life. That is what making mobility the center of longevity actually looks like.