FDA’s first mitochondria drug and the longevity playbook
The FDA’s September 2025 accelerated approval of Forzinity, the first mitochondria-targeted therapy, is a turning point. Here is how cardiolipin repair could scale from a rare disease win to near-term trials in aging muscles, hearts, and retinas—and what endpoints and biomarkers will actually matter.
Breaking through the membrane
On September 19, 2025, the U.S. Food and Drug Administration granted accelerated approval to Forzinity, an injectable form of elamipretide, for patients with Barth syndrome who weigh at least 30 kilograms. It is the first medicine cleared by the agency that is explicitly designed to target mitochondria, the power stations inside our cells. The authorization hinges on an improvement in knee extensor muscle strength, a surrogate that the agency judged reasonably likely to predict functional benefit. That single sentence in the approval notice matters far beyond a single ultra-rare disease. It signals that regulators are now willing to anchor decisions to direct measures of mitochondrial function and performance, not only to traditional disease endpoints. See the FDA approval announcement.
Barth syndrome is caused by mutations in a gene called TAZ that disrupt cardiolipin, a fatty molecule that helps form the inner scaffolding of mitochondria. Boys born with the disorder often face severe heart failure in infancy, and those who survive carry persistent muscle weakness and fatigue. Until now there was no approved therapy. Forzinity does not fix the gene. Instead, it supports the failing infrastructure inside mitochondria so that cells can make energy more reliably.
Cardiolipin, explained simply
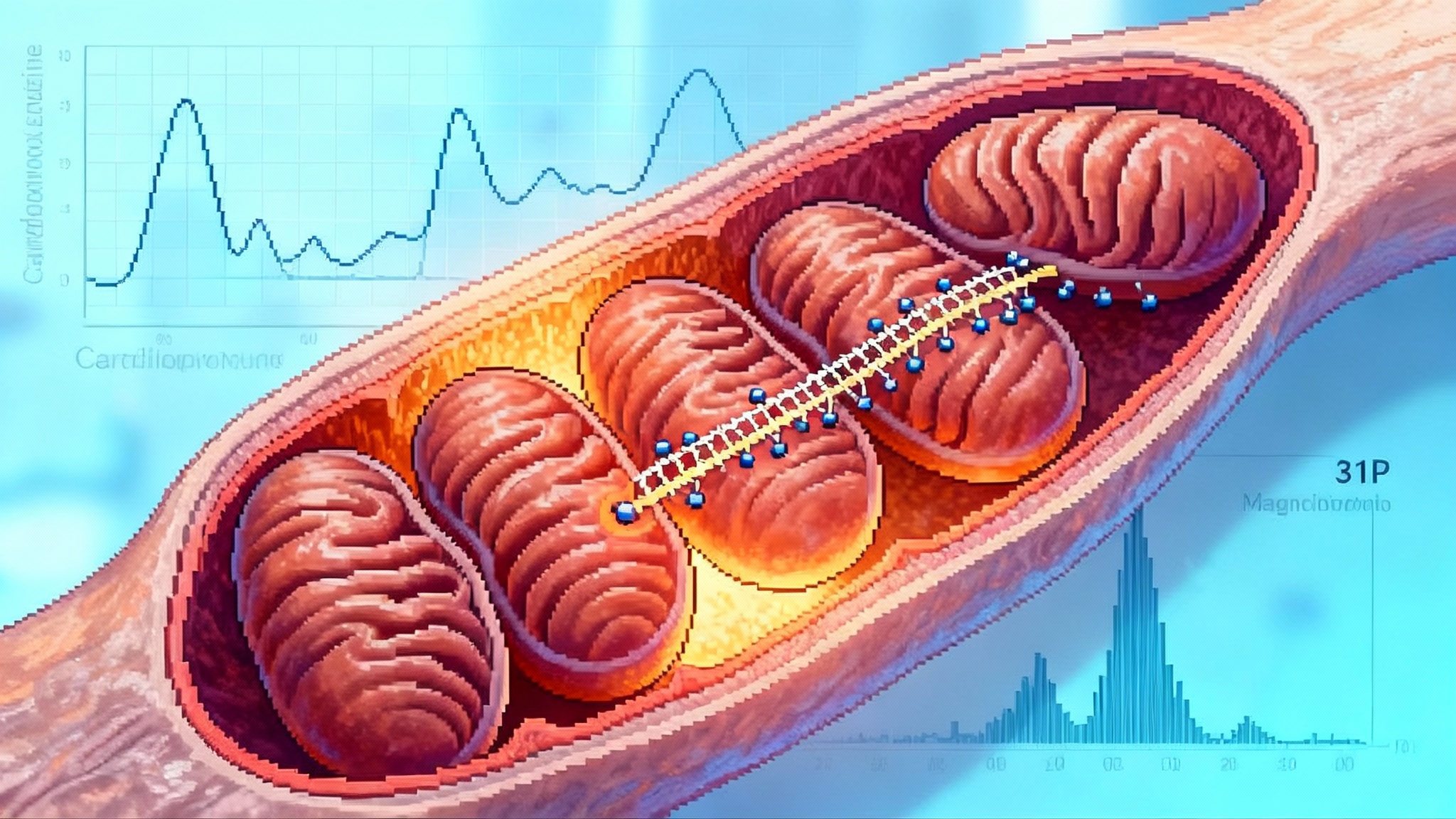
If mitochondria are power plants, cardiolipin is the rebar that holds the turbine room together. The inner mitochondrial membrane folds into narrow shelves called cristae. Along those shelves sit the protein complexes that shuttle electrons and pump protons, which together power the adenosine triphosphate turbine. Cardiolipin helps keep those shelves shaped correctly and keeps the complexes lined up so that the electron relay does not leak.
In Barth syndrome, cardiolipin quantity and quality are off. The cristae sag, the complexes lose alignment, and electrons slip away as heat and reactive oxygen. Elamipretide is a tiny synthetic peptide that carries a positive charge and an aromatic side chain that likes to sit next to cardiolipin. Think of it as Velcro for wobbly scaffolding. By binding to cardiolipin, elamipretide stabilizes the membrane, reduces leakiness, and supports the alignment of respiratory complexes. In cells and animal models, that stabilization improves electron flow, restores membrane potential, and can normalize the recovery of phosphocreatine after exertion. In people, the most direct readouts are increases in muscle strength or more efficient oxygen use during exercise.
Two practical takeaways follow from this mechanism. First, the drug is not disease specific. It targets a shared mitochondrial failure mode that appears in genetic disease, in common age-related disorders, and in the normal aging process. Second, the relevant biomarkers are functional, not only molecular. We care about ATP produced per unit of oxygen, about how quickly muscles recover after a sprint, and about whether cristae look organized under the microscope.
The regulatory drama and the price tag
Forzinity won under the accelerated approval pathway, which accepts a surrogate endpoint that is reasonably likely to predict benefit and requires a confirmatory postmarketing trial. That choice was not unanimous inside the agency. Several reviewers argued the clinical evidence was thin and that a prior pivotal study had missed its main goal. The agency’s leadership ultimately sided with the urgent unmet need and the consistency of strength gains and functional measures. The company has committed to a randomized, controlled confirmatory trial.
The second point of controversy is price. Reports indicate Forzinity will cost in the high six figures per year, with estimates approaching eight hundred thousand dollars for some patients. The company has announced a patient support program and says insurers will be engaged, but the numbers are stark. For a small population, the revenue may be necessary to fund additional trials. For the health system, it forces a debate about how to pay for platform therapies that start in ultra-rare diseases but aim to expand into larger indications. See recent regulatory debate and pricing coverage.
Why address the pricing head on in a story about longevity? Because what gets funded gets studied. If cardiolipin repair is to become a scalable strategy in aging, developers will have to prove benefit convincingly in common conditions and show value against standard of care. That means rigorous trials with practical endpoints, thought-through patient selection, and biomarker packages that shorten development cycles. For the broader reimbursement context, see our analysis of longevity infrastructure after weight loss.
From rare disease to geroscience
Geroscience asks whether slowing or reversing the hallmarks of aging can prevent disease. Mitochondrial decline is one of those hallmarks. It shows up as reduced ATP production, slower phosphocreatine recovery, impaired fat oxidation, and blunted peak oxygen consumption. By approving a mitochondria-targeted drug on a functional muscle endpoint, the agency has opened a door. As we noted in our review of longevity drug approvals in 2025, the bar for mechanism-linked function is rising. The question is how to walk through it responsibly in conditions that matter to millions.
Here are three near-term playbooks.
1) Sarcopenia and frailty
What to test: Older adults with low muscle mass and poor performance, but not yet disabled. A pragmatic cohort might be people over 70 with slow gait speed and a Short Physical Performance Battery score in the 4 to 9 range.
Primary endpoint: Improvement in knee extensor torque or isokinetic strength paired with six-minute walk distance, chosen to mirror the Forzinity precedent but broadened to track mobility. Secondary endpoints could include chair-stand time, timed up-and-go, and self-reported fatigue scores.
Biomarkers: 31P magnetic resonance spectroscopy to measure ATP flux and phosphocreatine recovery after standardized exercise; blood-based cardiolipin species as a signature of membrane quality; circulating cell respirometry to quantify oxygen consumption. Include cardiopulmonary exercise testing to capture VO2 kinetics during the onset of exertion, not only peak values.
Trial design: Twelve to sixteen weeks, double blind, with a run-in period to standardize protein intake and physical activity. Stratify by baseline mitochondrial function to see whether those with the worst cristae performance benefit more. Pre-specify a minimal clinically important difference on both strength and walking distance to align with payer expectations.
Why it matters: Frailty hospitalizations are expensive and common. If a mitochondria-targeted therapy can shift performance by amounts that translate to fewer falls or admissions, the value case becomes clearer.
2) Heart failure with preserved ejection fraction
What to test: Adults with heart failure symptoms and preserved ejection fraction who remain limited despite guideline-directed therapy. This group has impaired energetic reserve and abnormal skeletal muscle metabolism even when the heart pumps normally at rest.
Primary endpoint: Change in peak VO2 and Kansas City Cardiomyopathy Questionnaire overall summary. Secondary endpoints include ventilatory efficiency slope, daily step count from a wearable, and six-minute walk distance.
Biomarkers: VO2 on-kinetics during the first two minutes of exercise, skeletal muscle phosphocreatine recovery, and lactate threshold. Consider a substudy with lower-limb muscle biopsy for cristae ultrastructure and respiratory supercomplex assembly.
Trial design: Twenty-four weeks, multicenter, with a pragmatic home exercise program delivered to both arms to reduce confounding by activity.
Why it matters: Current drugs improve symptoms and reduce hospitalizations but many patients still struggle with exertion. A therapy that improves energetic efficiency could complement sodium glucose cotransporter 2 inhibitors and diuretics by directly addressing mitochondrial bottlenecks.
3) Retinal aging and early macular degeneration
What to test: Adults with early to intermediate age-related macular degeneration or age-related decline in contrast sensitivity. Retinal pigment epithelium is highly energy intensive, and mitochondrial dysfunction is implicated in photoreceptor vulnerability.
Primary endpoint: Low-luminance visual acuity or contrast sensitivity function. Secondary endpoints might include microperimetry and dark adaptation recovery time.
Biomarkers: Optical coherence tomography metrics of photoreceptor integrity, retinal oximetry, and mitochondrial DNA damage markers in circulating extracellular vesicles. A small imaging substudy could quantify retinal oxygen extraction during flicker stimulation.
Trial design: Six to nine months with monthly visits, leveraging sensitive functional tests that move before structural degeneration advances.
Why it matters: Preventing or slowing early decline in visual function is a meaningful win for independence in older adults and can be detected before major anatomic changes.
What counts as a good biomarker now
The new approval clarifies which mitochondria-relevant measures can carry regulatory weight and which are best as supportive.
- ATP flux and recovery: 31P magnetic resonance spectroscopy provides a direct read on energetic recovery after standardized work. It is quantifiable, repeatable, and maps to real-world function.
- Cardiolipin signatures: Targeted lipidomics can measure total cardiolipin and the ratio of mature to immature species. In mitochondrial disease these profiles are skewed. In aging, subtle shifts may correlate with performance and may normalize with therapy.
- VO2 kinetics: Traditional peak VO2 misses early inefficiencies. Measuring oxygen uptake during the first two minutes of exertion reveals how quickly mitochondria respond to demand, which is often the limiting factor in older adults.
- Portable surrogates: Handgrip strength, chair-stands, and six-minute walk distance are inexpensive and correlates of independence. None proves mitochondrial repair alone, but together with energetic biomarkers they create a credible package.
The rule of thumb is simple. Pair a functional readout that a person feels with a mechanistic readout that explains why it changed. That pairing is the shortest path from signal to approval.
Who moves next, and with what
Next generation peptides: Elamipretide is the best known of the Szeto-Schiller family of peptides that bind to cardiolipin. Developers are testing variants tuned for central nervous system exposure or greater membrane residence time. Expect at least one readout in neurodegenerative or amyloid-associated cardiomyopathy settings in 2026. Watch for molecules that improve cristae curvature and supercomplex assembly rather than only antioxidant effects.
Mitophagy and autophagy boosters: Urolithin A has already shown improvements in leg muscle endurance in older adults and is being explored alongside exercise. Low dose rapamycin analogs and spermidine are in small trials that track strength and infection outcomes, with mechanistic markers that include autophagic flux and mitochondrial biogenesis. The most compelling programs will combine a clearance booster with a cristae stabilizer on the theory that better quality control plus better structure yields larger, more durable gains.
Metabolic reprogrammers: Peroxisome proliferator-activated receptor delta agonists and nicotinamide adenine dinucleotide strategies are adjacent. Their success depends on whether they can raise the ceiling for mitochondrial output without inducing inefficiency or arrhythmia. For background on clinical development constraints, see our overview of NAD+ restoration programs.
Diagnostics and monitoring: Expect a push to standardize cardiolipin assays from research labs into clinical reference labs, and to bundle 31P magnetic resonance spectroscopy into multi-site trials with harmonized exercise protocols. Wearables will matter, not as toys, but as continuous measures of energy use and recovery timing.
Practical implications for 2026
For biotechs: Design trials that put mitochondria at the center, not the periphery. Start with a functional endpoint a payer will recognize, and earn the right to explore broader mechanism inside the same protocol. Build an assay plan that includes ATP flux, cardiolipin signatures, and VO2 kinetics, and predefine how each will support dose selection or subgroup enrichment.
For clinicians: Expect patient questions. Forzinity is approved only for Barth syndrome. Off-label use in aging is not warranted without evidence. That said, routine care can lean more on mitochondrial literacy. Order cardiopulmonary exercise testing to unmask early inefficiency in breathless older adults. Track simple performance measures alongside disease metrics to see whether a therapy changes how patients live, not only how they scan.
For payers and policy makers: The accelerated approval sets a precedent that can shorten timelines for mitochondria-targeted drugs in common diseases. Tie reimbursement to confirmatory outcomes and invest in registry infrastructure that captures functional and mechanistic markers, not only hospitalizations. For a related systems view, revisit our primer on longevity infrastructure after weight loss.
A 2026 watchlist
- Cardiolipin stabilizers beyond elamipretide in cardiac and neurodegenerative indications, with at least one readout that pairs VO2 kinetics and quality-of-life scores.
- Mitophagy boosters in sarcopenia that combine functional endpoints with 31P magnetic resonance spectroscopy and muscle ultrasound. A positive result here would change standard preventive care.
- Retinal trials that move first on contrast sensitivity and dark adaptation, not only lesion size, while adding mitochondrial biomarkers to increase confidence in the mechanism.
- Standardized cardiolipin panels in blood and dried blood spots, opening the door to screening in large cohorts.
- Pragmatic heart failure trials that layer a mitochondria-targeted therapy on top of sodium glucose cotransporter 2 inhibitors, seeking additive gains in effort tolerance.
The bigger picture
The first mitochondria-targeted approval did not arrive through a blockbuster cardiovascular trial or a sweeping aging study. It came through a small community, measured with a careful functional test, and pushed across the line by a mechanistic story that made sense. That is both humbling and instructive. If we want mitochondrial repair to scale as a longevity strategy, we should take the same path on purpose. Start where the biology is clearest. Measure what actually powers a life, not only what shows on a scan. Build programs that pair function with mechanism, and prices with proof.
Mitochondria were once an afterthought in drug development. With Forzinity, they are on the main stage. The next act belongs to those who can turn a fragile scaffolding into stronger everyday performance for millions, one well-chosen endpoint at a time.