Gene Edited Pig Kidneys Enter First U.S. Clinical Trials in 2025
The FDA has cleared the first U.S. human trials of gene-edited pig kidneys. We unpack the 10 to 69 genomic edits, the new anti-rejection regimens, and the biosecure pig herds that could finally end the kidney shortage.
The starting gun for a new kind of transplant
This year, a line that once separated science fiction from hospital routine moved. The U.S. Food and Drug Administration cleared the first human trials of gene-edited pig kidneys, and the first patient in United Therapeutics’ UKidney study has already been transplanted at NYU Langone. United Therapeutics announced FDA clearance of the UKidney trial alongside the initial surgery.
In parallel, eGenesis, whose team supplied the gene-edited kidney for the first living recipient in 2024, secured IND clearance for EGEN-2784 to begin its own phase 1/2/3 trial later in 2025. Their program builds on expanded-access cases that extended pig kidney function in people for months, providing the clinical breadcrumbs regulators look for before they authorize formal enrollment.
Two companies, two trial designs, one practical goal: show that a pig kidney can work reliably in people who would otherwise spend years on dialysis or die waiting for a human organ.
Why the edits matter: from 10 to 69
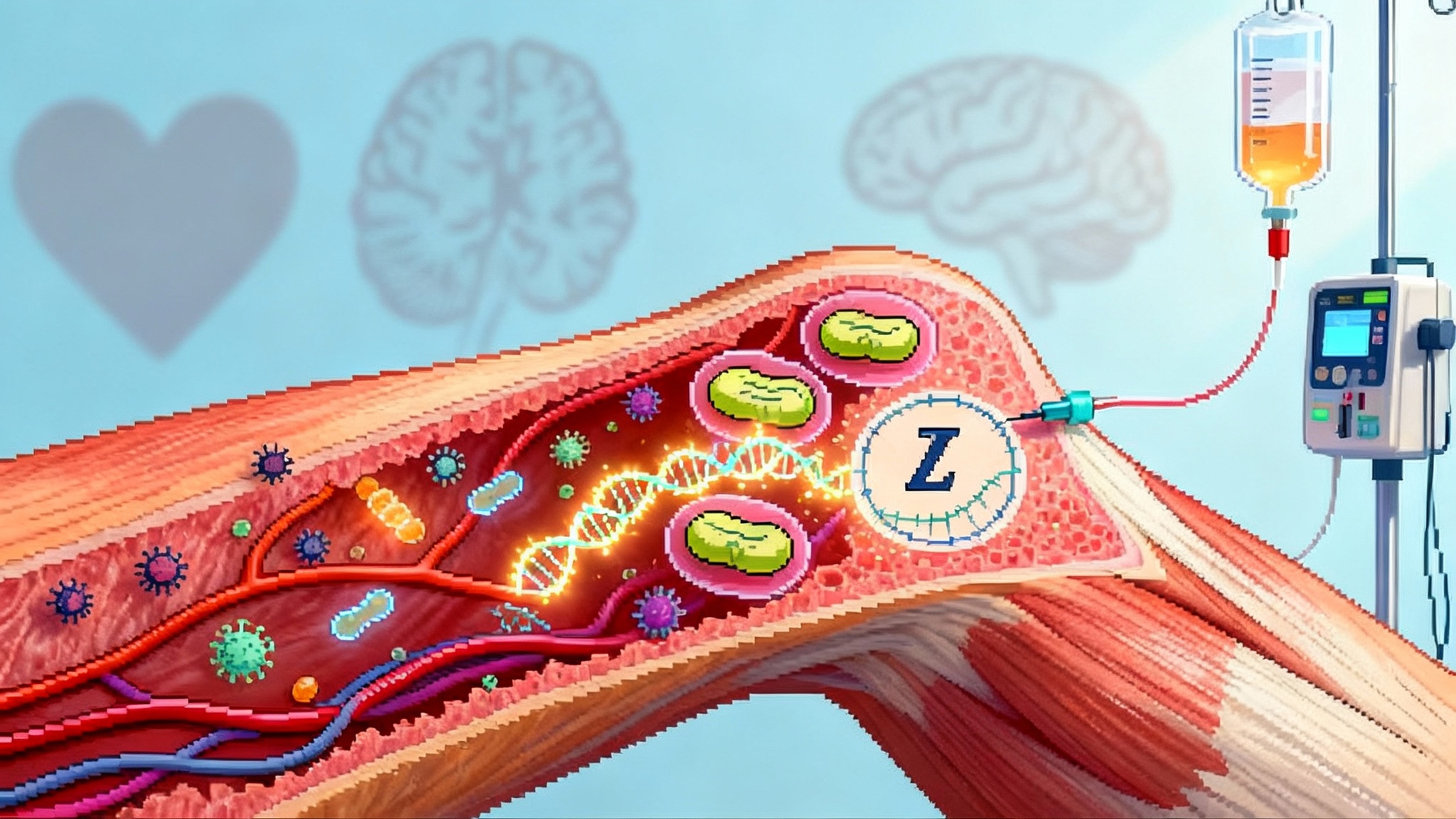
A xenograft faces three categories of problems the moment it meets human blood. First is hyperacute rejection, driven by preformed antibodies that recognize sugary decorations on pig blood vessel cells. Second is a cascade of complement activation and clotting that batters the organ’s microvasculature. Third is the ongoing cellular immune response, where T cells and other immune players treat the graft like an invaded fortress.
The edit counts you see in headlines tell you which of those problems a company is taking on and how aggressively.
- United Therapeutics’ UKidney design lists 10 edits. Six are human genes added to the pig genome to make its blood vessels look and behave more like ours. Think of these as coat checks that let human complement and clotting systems pass without starting a brawl. The remaining edits remove or alter pig genes that present the most provocative targets to human antibodies. The strategy is minimalist by xenotransplant standards, on purpose. Fewer edits can mean simpler quality control for each donor pig, fewer ways to disrupt normal kidney biology, and a cleaner regulatory package.
- eGenesis talks about 69 edits. That larger number reflects three buckets of changes: removing the glycan antigens that trigger hyperacute rejection, inserting a suite of human immune-regulatory genes, and, crucially, inactivating dozens of copies of porcine endogenous retroviruses embedded in the pig genome. If the UKidney is a tight toolkit aimed at the known troublemakers, EGEN-2784 is a fully remodeled house with new insulation, wiring, and a firewall in the basement.
Both approaches are coherent. This is not a numbers contest. It is two engineering philosophies converging on the same performance target: a kidney that filters blood, makes urine, balances electrolytes, and does not invite clotting, inflammation, or runaway immune attacks. The underlying biology reads like a checklist the organ must pass every hour: do red blood cells flow without sticking, do complement proteins glide by without drilling holes in endothelium, do platelets mind their own business, and does the immune system stand down when it sniffs a foreign surface that nonetheless signals do not attack.
For readers tracking gene editing across modalities, see how clinical editing strategies mature in our coverage of PCSK9 base editing progress.
The new anti-rejection playbook
Human kidney transplants rely on drugs that mute T cells and blunt antibody production. Xenografts need all that and more, because the pig–human molecular mismatches are broader and louder. The playbook taking shape in 2025 has three notable elements.
-
Costimulation blockade. Instead of hammering T cells across the board, newer antibodies interrupt the specific handshake signals T cells need to fully activate. This is like removing the spark plug instead of turning off the whole engine. It reduces the risk of early, aggressive cellular rejection while avoiding some toxicities of older regimens.
-
Complement control. Complement proteins are part of innate immunity and can be ruthless toward foreign surfaces. Clinical teams are adding targeted complement inhibitors to protect the pig kidney’s delicate inner lining, especially in the first weeks. That lining is where rejection and clotting tend to reinforce each other, so keeping it calm buys the graft time to adapt.
-
Anticoagulation tuned to the graft. Pig and human coagulation factors do not match perfectly. Trial protocols use careful anticoagulation and antiplatelet strategies that are adjusted as the graft shows it can maintain smooth flow. The goal is to avoid both the smoldering clots that choke a graft and the bleeding that comes from overcorrection. In practical terms, that means tight lab monitoring in the first month and preplanned tapering if early biopsies and blood tests look stable.
Layered on top is vigilant infection surveillance. The biosecure breeding facilities that produce donor pigs are screened for known pathogens, and the genetically inactivated endogenous retroviruses are a backstop. Still, recipients will be followed for years, with routine polymerase chain reaction testing and public health coordination to ensure that a theoretical cross-species infection does not slip past the radar. For a broader lens on immune calibration with aging, see thymus revival and immune aging.
Manufacturing a reliable organ supply
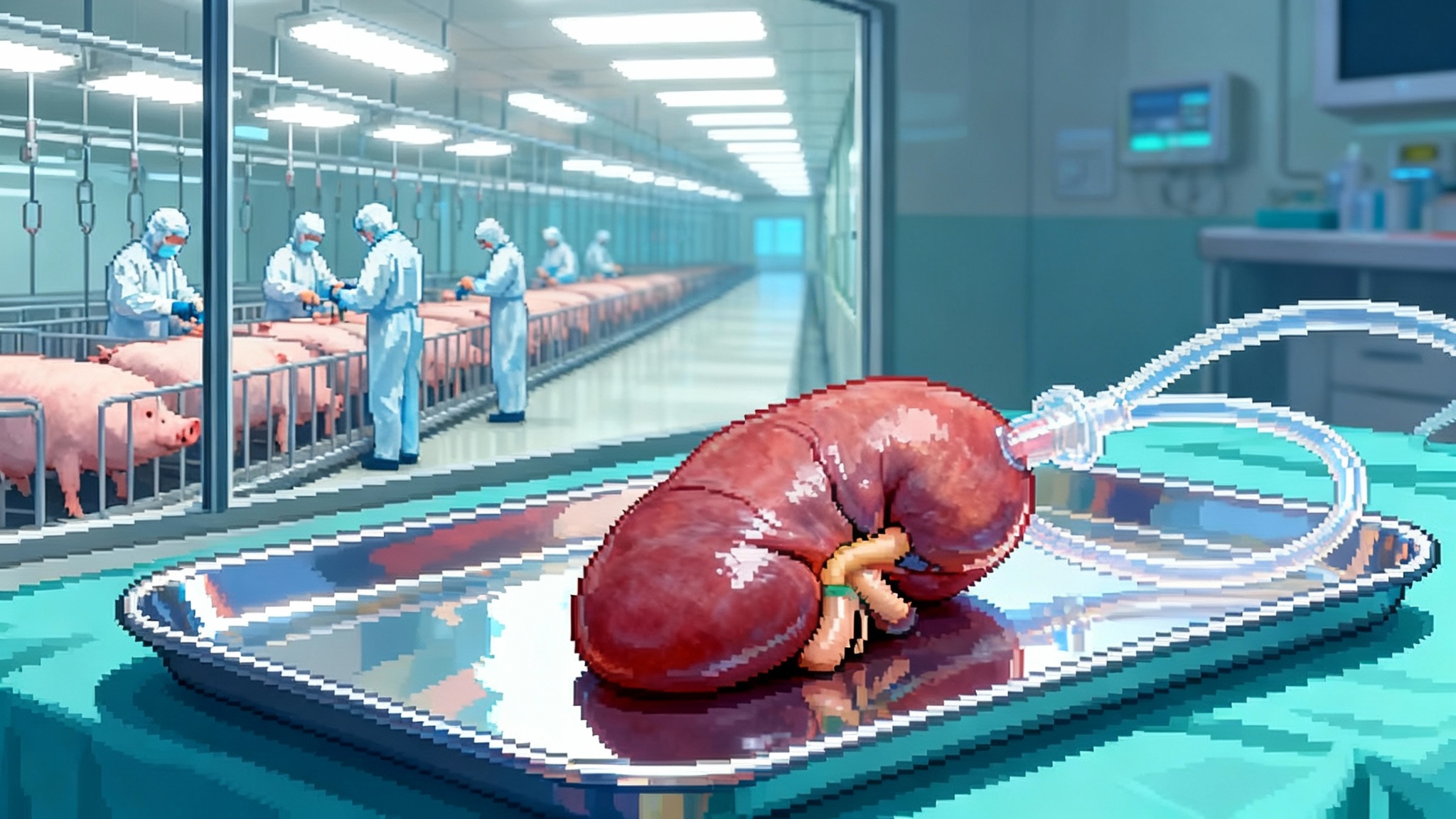
A pig kidney is not a small-molecule drug, and it is not a lab-grown cell therapy. To scale xenografts, companies have to stand up something closer to a pharmaceutical farm, with the quality systems of a biologics plant and the biocontainment of a vaccine factory.
Here is what that looks like behind the scenes.
- Designated pathogen-free herds. Donor pigs are raised in barrier facilities that control air, water, feed, and personnel contact. Cesarean delivery and early-life isolation reduce vertical transmission of pathogens. Every animal is tracked like a lot number in a biologics run.
- Genetic identity and integrity. If you promise six human transgenes and three antigen knockouts, you have to prove every donor pig actually has them, in the right places, with no unintended edits. That means whole-genome testing for each breeding line, routine expression assays for the human transgenes, and release criteria that look more like a batch record than a barn report.
- Crossmatch as a new logistics step. Instead of waiting for a scarce human organ and rushing to find a compatible recipient, xenotransplant centers can pre-crossmatch patients against a panel of donor pigs. That enables scheduled surgeries, better staffing, and less cold ischemia time, which is a quiet killer of transplant outcomes. It also turns procurement from a midnight dash into an industrial handoff.
- Organ transport and preservation. Pig kidneys tolerate established human preservation techniques, but timelines will be optimized for shorter distances between farms and transplant centers. Expect to see two models emerge: regional herds that serve clusters of hospitals, and national hubs that can fly organs under tight temperature and time controls. Related technology is advancing quickly; see nanowarming organ banking advances.
- Cost curves and capacity. Dialysis in the United States costs on the order of tens of billions annually. A single transplant plus medications is cheaper over a few years than chronic dialysis, but the initial cost of a first-generation xenograft will be high. Companies will push costs down by standardizing donors, improving surgical predictability, and moving from pilot herds to larger, modular facilities. Insurers and Medicare will ask for real outcomes data before coverage expands. Trials designed for registration are the bridge to that coverage.
What happens in the next 12 to 24 months
Regulatory timelines are often abstract, so here is a concrete map of what to watch, pegged to what the companies have already disclosed.
- First six UKidney patients complete 24-week follow-up. United Therapeutics’ initial cohort is staged with a built-in 12-week pause between the first and second surgeries. Expect early safety updates and basic kidney function data at three and six months after the first few transplants, followed by a Data Monitoring Committee review. If the signal supports expansion, the study can grow toward as many as 50 participants across additional centers, with a focus on consistent graft function at 24 weeks and an acceptable safety profile.
- eGenesis opens enrollment under its IND. The company’s expanded-access cases provided durable function in real patients, which is exactly the on-ramp regulators look for. The IND allows a structured trial with defined endpoints and broader inclusion criteria. As the first cohorts reach 24 weeks, look for convergence or divergence with the UKidney experience on rejection rates, complement use, and anticoagulation strategy.
- Chemistry, Manufacturing, and Controls mature. For both programs, 2025 and 2026 are the years when the manufacturing playbook hardens. Expect more detail on herd scale, genetic release testing, and site-to-site reproducibility, because a biologics license application lives or dies on whether the product is the same every time.
- Pre-BLA dialogues if signals hold. If a half dozen to a dozen patients show sustained graft survival at 24 weeks with manageable adverse events, sponsors will seek formal meetings with the FDA to scope a registration path. That path could include expansion to a target sample size, a rolling review of manufacturing sections, and post-marketing surveillance commitments for infectious-disease monitoring and long-term graft outcomes.
- Coverage conversations begin. Payers will want to see hospital length of stay, readmission rates, and drug intensity over the first year versus dialysis costs. Medicare has the largest stake. The agency will press for data on functional status, return to work, and quality of life in the months after surgery.
Taken together, those milestones add up to a plausible route to a biologics license application in preparation within about two years if the data cooperate, with the first limited commercial availability possible on a center-by-center basis soon after. That is not a promise, it is a map readers can use to track progress and pressure test claims as updates arrive.
The impact if xenografts scale
The organ shortage is not a talking point. As of early 2025, roughly half a million Americans receive dialysis, and the national kidney waitlist hovers around ninety thousand names. Twelve people die on an average day in the United States while waiting for a transplant. The arithmetic behind those losses is simple. Human kidneys are scarce, and many do not match the blood type or immunologic profile of the person who needs one.
A reliable pig kidney supply would rewrite that arithmetic in three ways.
-
Replace years on dialysis with scheduled surgery. Instead of waiting for a phone call, patients could be crossmatched, optimized, and transplanted on a planned date. That alone reduces complications, because long dialysis exposure increases cardiovascular risk and wears down vascular access, making future surgery harder.
-
Reduce deaths linked to access and geography. Dialysis access and transplant referral vary by region and by socioeconomic status. An on-demand organ source lowers the penalty for living far from a regional transplant hub and expands the set of patients a center can confidently accept. The earliest trials target older patients, ages roughly 55 to 70, who have been on dialysis for at least six months and may be less likely to receive a human organ in time. If outcomes are strong in that group, eligibility will broaden.
-
Reframe longevity care. A functioning transplant does not just add years, it gives back hours every week. Dialysis ties people to a chair or a machine. A xenograft that performs like a standard transplant would mean fewer hospitalizations, more mobility, and more energy to engage in the preventive care that compounds health over time.
There will be tradeoffs. Recipients will need immunosuppression with its infection risks. They will undergo frequent blood tests and biopsies early on. Some grafts will fail. Surgeons will learn what warning signs precede rejection in this new setting and how to reverse them faster. But compared with the certainty of dialysis-related mortality and the uncertainty of human organ availability, the equation could tilt quickly if a majority of trial recipients hit six months with good function.
What hospitals, payers, and policymakers can do now
- Hospitals should stand up cross-functional xenotransplant teams today. That means bringing transplant surgeons, nephrologists, infectious disease physicians, pharmacists, and critical care under one operating model, with an accountable lead and a 24-week post-surgical playbook that everyone signs. Identify which patients would be candidates in the first two years, and build education materials for them and their families.
- Payers should define outcomes that trigger reimbursement expansion. Be specific: 24-week death-censored graft survival, hospital length of stay, readmissions within 30 and 90 days, and steroid exposure in the first month. Publish the bar now so centers and sponsors know what data to collect.
- Policymakers should prebuild the surveillance net. Long-term public health monitoring for zoonoses is not optional. Fund a centralized registry with standardized polymerase chain reaction testing protocols and data sharing between transplant centers and state health departments.
- Employers and philanthropies should invest in patient navigation. The first wave of recipients will need dense support. A nurse navigator and social worker can prevent the small failures that cascade into readmissions, from missed labs to delayed drug refills.
The health equity lens
Organ scarcity amplifies inequity. People who lack flexible jobs, transportation, or paid leave are less likely to complete evaluation and stay eligible for a human organ. Xenografts change some of those constraints. Scheduled surgeries can be booked when support is available. Pre-crossmatching reduces wasted trips. If companies and centers price fairly and design access programs that mirror the best oncology models, xenotransplantation can narrow gaps rather than widen them.
The wrong version of this future is easy to imagine, where only a few hospitals offer the procedure and early costs put it out of reach. The right version is attainable: a covered benefit with clear inclusion criteria, transportation support for frequent early follow-ups, and a registry that reports outcomes by demographic variables so problems are visible and solvable.
What to watch next
Three signals will tell you if this field is on track.
- The six-month curve. Do most grafts make it to 24 weeks with stable creatinine, minimal proteinuria, and no major thrombotic events. That is the trial’s first gate.
- Drug tapering. Can teams reduce intensity of complement inhibitors and anticoagulation without awakening rejection or clotting. That would lower cost and side effects.
- Manufacturing reproducibility. Do different centers and different donor herds generate similar results. A biologics license application depends as much on showing a consistent product as on headline survival.
The bottom line
A kidney does quiet, essential work. It keeps blood chemistry in a narrow band where life thrives. For more than half a million Americans on dialysis, that work happens by machine, at a high cost in time and health. In 2025, for the first time, there is a credible path to replacing that machine with a living organ from a biosecure herd.
The science is disciplined. The regulatory path is sketched. The first surgeries are done. Over the next two years, xenotransplantation will either clear its 24-week bar in enough patients to justify a biologics license push, or it will stall and return to the lab. My bet is that the combination of focused gene edits, smarter immune control, and industrial-scale manufacturing will carry it over the line.
If you want a single sentence to track the story, use this: when a pig kidney can reliably give a human six months of good function, scheduled on a calendar instead of chanced on a phone call, the organ shortage starts to end.