Plasma Exchange Rewrites Aging: Inside the 2025 Human Trial
In May 2025, Aging Cell reported the first randomized human study suggesting that therapeutic plasma exchange, especially when paired with intravenous immunoglobulin, can lower biological age in older adults. Here is what changed, what it costs, who benefits first, and the realistic path to 2028.
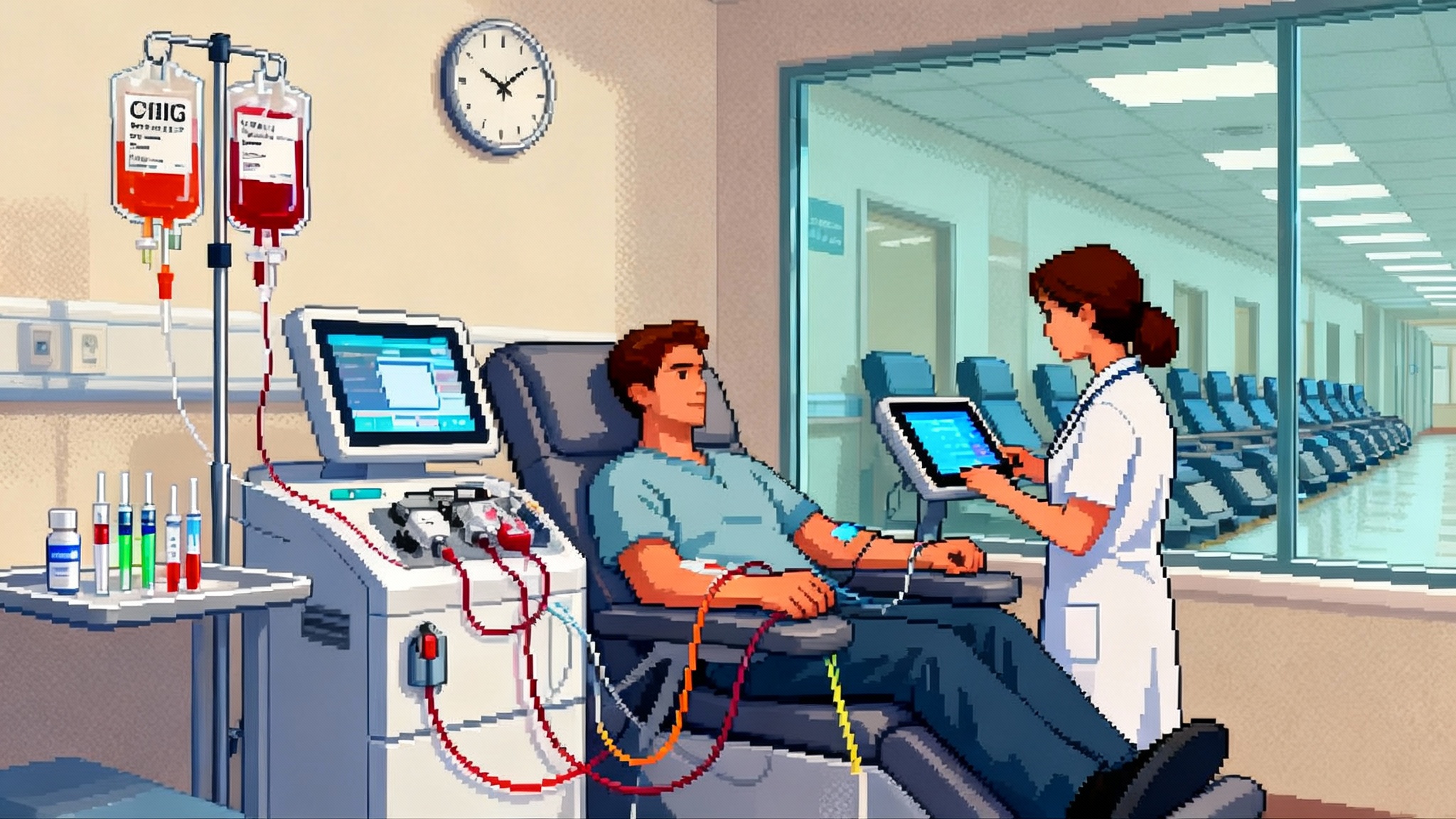
Breaking: A controlled human study nudges biological age backward
On May 27, 2025, Aging Cell published the first randomized, single-blind, placebo-controlled human trial showing that therapeutic plasma exchange can shift biological age. The addition of intravenous immunoglobulin made the effect larger. In a cohort of older adults, the combination produced a multi-omics measured rejuvenation on par with a couple of calendar years. You can read the peer-reviewed summary in the Aging Cell randomized trial.
That sentence lands differently because it moves the conversation from mice to people, and from ideas to evidence. The result is not longevity as a promise. It is a measurable change in biological clocks and immune markers after a medical procedure that is already in hospital workflows.
From parabiosis headlines to protein remodeling
A decade of animal studies teased a provocative idea. When researchers stitched the circulatory systems of young and old mice together, or simply diluted old plasma and replaced it with albumin-containing solutions, the old tissues acted younger. The common thread was not magic in young blood. It was the removal and replacement of circulating proteins that accumulate with age.
Therapeutic plasma exchange, or TPE, is that filtration and refresh step for humans. During TPE, the cellular part of blood is returned while the plasma is removed and replaced with sterile solutions, usually albumin and saline. Intravenous immunoglobulin, or IVIG, is a pooled antibody therapy that can be given after TPE to stabilize the immune system and blunt rebound inflammation. In clinical medicine these tools treat Guillain-Barré syndrome, myasthenia gravis, thrombotic thrombocytopenic purpura, and other immune-driven illnesses. The 2025 trial asked a new question: what happens if you use TPE, with or without IVIG, in generally healthy older adults and then measure aging biology across thousands of molecules and cells. This protein remodeling frame sits alongside efforts to reset the thymus to restore immunity.
What the 2025 trial actually did and found
The study randomized 42 participants over age 50 into several arms. The key comparisons were biweekly TPE, biweekly TPE plus IVIG, monthly TPE, and placebo. The researchers tracked epigenetic clocks, proteomics, metabolomics, glycomics, cytokines, and immune cell composition, then rolled those changes into biological age estimates.
The headline findings were clear:
- TPE reduced biological age markers versus placebo, and the effect was largest in the biweekly TPE plus IVIG arm.
- On average, participants in the TPE plus IVIG arm saw about a 2.6-year reduction in biological age, while TPE alone landed closer to 1.3 years.
- People who started with worse metabolic and liver markers benefited most, which hints at a precision medicine strategy rather than a one-size-fits-all protocol.
- The first three sessions delivered the biggest gains, then returns diminished, which is a practical clue for dosing cadence.
- Safety looked acceptable in this small study, with two discontinuations over months of treatment and one event related to IVIG, and no pattern of severe procedure-related complications.
This is not immortality. It is a biological nudge that aligns with a plausible mechanism, measured by multiple independent assays, delivered through a regulated procedure that already has staff, codes, and machines.
What protein remodeling means in practice
Think of the plasma proteome as the soundtrack of inflammation and repair. As we age, pro-inflammatory notes grow louder and anti-inflammatory harmonies fade. TPE removes a swath of that soundtrack at once, which drops the volume on proteins that drive chronic inflammation. IVIG then adds a stabilizing chorus, saturating immune receptors and helping the system reset to a calmer baseline. The trial’s multi-omics readouts captured that shift, with coordinated changes in senescence-associated proteins and immune cell composition. Similar organ-focused strategies, such as liver tropic senolytics for MASLD, point to a broader toolkit for remodeling damaged systems.
From biomarker wins to hard outcomes
Biological clocks are useful, but payers and regulators will ask about outcomes that matter directly to patients.
- Durability: How long do the gains last after the early sessions. The study suggests most of the drop arrives early, then plateaus. We need follow-up through 6, 12, and 24 months to map the rebound curve.
- Function: Do gait speed, grip strength, balance, and stair climb improve, and do those gains beat well-designed placebo controls. The study reported signals on balance and strength. Larger samples must confirm.
- Morbidity: Do rates of respiratory infections, autoimmune flares, or hospitalization decline over the following year. These endpoints link directly to quality of life and medical cost.
- Mortality: Not immediately feasible, but composite outcomes, such as time to first serious infection or fall, can serve as near-term surrogates.
If protein remodeling only moves clocks for a few months, then the clinical service becomes a periodic tune-up. If it bends infection curves and maintains muscle power across seasons, then it becomes part of prevention guidelines for select populations. The stakes echo other care innovations that aim to shift healthspan, such as xenotransplant trials shifting healthspan.
Dosing cadence and protocol design
The 2025 study compared biweekly and monthly schedules and observed diminishing returns after the first three sessions. That points to a practical regimen that starts with an induction phase of three exchanges over six weeks, followed by a less frequent maintenance schedule guided by biomarkers and function tests. IVIG appears to amplify and stabilize benefits in those with higher baseline inflammatory load, yet it adds cost and potential infusion-related side effects. A precision protocol might therefore include IVIG for individuals with elevated inflammatory proteins, poor glycemic measures, or specific autoantibodies, while omitting IVIG for lower-risk patients.
Key design features for clinics:
- Baseline panel: epigenetic age, inflammatory proteomics, glycan age, high-sensitivity C-reactive protein, liver enzymes, fasting glucose, and a frailty index.
- Induction: three TPE sessions over six weeks, consider IVIG after each session for high-risk profiles.
- Maintenance: quarterly reassessment, additional exchange only if biological age or inflammatory markers drift above preset thresholds or if functional scores slip.
- Safety guardrails: calcium monitoring during exchange, immunoglobulin levels if repeated IVIG is used, and strict infection screening between sessions.
Safety, logistics, and cost
Therapeutic plasma exchange is an invasive but routine hospital procedure. Common side effects include transient hypotension, tingling from low calcium, and fatigue. Serious complications are uncommon in experienced centers, but the risk is not zero. IVIG can cause headache, infusion reactions, and rarely thrombosis or renal stress, particularly at higher doses.
Costs vary widely by region and site of care. A single TPE session in the United States is typically billed in the low to mid four figures before facility fees. IVIG adds material cost that scales with body weight and dose, and can be several thousand dollars or more per infusion. These are order-of-magnitude numbers, not quotes, and they underscore why payer policy will shape access.
Operationally, capacity is real. TPE requires trained nurses, an apheresis physician or defined oversight protocol, and devices such as the Spectra Optia or similar platforms. Albumin and IVIG supply chains run through a few manufacturers. Any broadening of indications must not strain availability for life-saving uses.
Who could benefit first
Evidence will likely concentrate first in three groups where both biology and clinical needs align.
- Frailty and pre-frailty: Older adults with elevated inflammatory profiles, slower gait speed, and recurrent infections. The near-term goal is fewer falls, fewer hospitalizations, and better physical performance.
- Post-chemotherapy recovery: Patients whose immune systems are slow to rebound after cytotoxic regimens. Protein remodeling may speed normalization of inflammatory proteins and improve strength, while careful monitoring avoids immunosuppression.
- Autoimmune conditions in remission: Individuals with high inflammatory markers despite symptom control. TPE is already used for flares in several diseases, so a maintenance-style protocol could target relapse prevention in carefully selected cases under specialist oversight.
High-performance but healthy biohackers are not the early priority. Safety margins and scarce resources favor those with clear medical need and tractable endpoints.
Payer pathways and realistic access
Coverage today follows established indications. TPE is generally reimbursed when used for conditions supported by specialty society guidelines and payer policies. It is not covered for anti-aging. The path to broader access runs through outcomes, not marketing.
Practical moves for 2026 and 2027:
- Coverage with evidence development for frailty pilots, where Medicare Advantage plans and integrated systems test induction plus maintenance protocols tied to falls, infection rates, and functional scores.
- Bundled payment experiments that include lab panels, the exchange sessions, and home-based functional testing, thereby limiting cost surprises for both plans and patients.
- Prior authorization templates that require baseline biomarker thresholds and structured follow-up.
If trials show durable reductions in hospital days or infections, actuaries will find room in benefits. If effects are short lived or limited to clocks alone, coverage will stay narrow.
The near-term trial agenda
To move from biomarker wins to regulated longevity services by 2028, the field needs a staged program with clear endpoints and statistical power.
- Phase 2b, frailty focused, 2026 start: multicenter, 300 to 500 participants age 70 plus with elevated inflammatory markers and slow gait speed. Arms: placebo, TPE, TPE plus IVIG, induction then maintenance guided by biomarkers. Primary endpoint: change in Short Physical Performance Battery and falls over 12 months. Key secondary: infection-related urgent care visits, hospitalizations, and quality of life.
- Post-chemotherapy recovery, 2026 start: 200 patients in hematology and solid tumor clinics. Endpoint: time to neutrophil and functional recovery, days to return to baseline activity, infection rate at 90 and 180 days.
- Autoimmune remission maintenance, 2027 start: myasthenia gravis and vasculitis cohorts in stable remission. Endpoint: time to flare, steroid exposure, and patient-reported function.
- Durability registry, continuous: real-world tracking of biological age and proteomic markers at 3, 6, 12, and 24 months post induction to pin down relapse curves and resource use. The Buck Institute study is already a registered trial NCT06534450.
All interventional trials should be registered and set up to allow adaptive dosing decisions based on biomarker drift.
Building the clinical infrastructure by 2028
If the data hold, we will need a safe and scalable delivery model that respects existing patients who need TPE for acute disease while opening access for prevention and recovery.
- Equipment and sites: expand capacity in hospital apheresis units and develop accredited ambulatory centers with clear escalation pathways. Partner with device makers and plasma product suppliers to forecast demand and avoid shortages.
- Workforce: cross-train dialysis and infusion nurses on TPE and IVIG protocols. Build standardized competency modules and simulation drills for adverse events.
- Data layer: integrate epigenetic age, proteomics scores, and functional testing into electronic records. Create a shared registry to track outcomes and refine algorithms that predict who benefits and when to retreat.
- Supply assurance: negotiate guaranteed albumin and IVIG volumes with manufacturers through group purchasing organizations. Add ethical allocation rules to protect acute indications if demand spikes.
- Patient experience: design six-week induction pathways with transportation, light meals, and at-home monitoring kits. Every step should be boring and safe, not boutique and exclusive.
Cautious optimism and clear pitfalls
The study is small, and biological clocks can be noisy. Placebo controls, blinding, and converging molecular signals strengthen the case, yet single-digit dozens of participants cannot answer long-term questions. Conflicts of interest exist, including company involvement, which is disclosed. That is common in translational work, and it increases the need for independent replication.
There is also the equity problem. If plasma-based protein remodeling becomes a boutique service for the wealthy, it will stall. The right path is hospital-grade delivery, benefit designs that reward prevented hospital days, and protocols that gate access to those with clear biomarkers and functional risk.
Finally, the mechanism matters. If we are simply turning down inflammatory proteins for a short while, then we need to know the best way to combine TPE with other interventions like resistance training, vaccination scheduling, sleep optimization, and metabolic care to lock in the gains.
What to do next, and why
- For clinicians: consider referring older adults with high inflammatory markers into upcoming trials. The risk profile of TPE is familiar, and the learning value is high.
- For health systems: stand up a small protein remodeling clinic that runs within existing apheresis infrastructure. Start with a protocolized induction phase and a registry. Measure function and infections, not just clocks.
- For payers: fund coverage-with-evidence pilots in frailty and post-chemotherapy recovery. Tie payments to falls, infection rates, and hospital days. Define stop rules if signals fail to appear by six months.
- For researchers: replicate the multi-omics framework and share data to test predictive signatures. Pre-register durability analyses and adaptive maintenance dosing rules.
- For patients: do not chase unregulated offerings. Ask about trials, ask about risks, and remember that exercise and muscle remain the foundation even as protein remodeling matures.
The signal is real, the service is not yet
The 2025 Aging Cell trial shows that plasma-based protein remodeling can move objective aging biology in humans, with the largest gains when IVIG accompanies exchange. That is enough to justify serious follow-on trials, not enough to justify a clinical land rush. If the next two years deliver function, fewer infections, and durable gains, then by 2028 we can talk about regulated longevity as a routine service. Until then, cautious optimism is the right stance. The bloodstream’s soundtrack can be retuned, and now we must learn how to make the music last.