Pig Kidneys Go Clinical: The 2025 Xenotransplant Pivot
2025 marked the year xenotransplantation left the lab. The FDA cleared the first human trials of gene-edited pig kidneys, and one recipient lived nearly nine months with a single graft. Here is how trials, safety guardrails, and manufacturing are turning a moonshot into medicine.
The year pig kidneys left the lab
If you follow organ transplant news, 2025 felt different from every year before it. In February the Food and Drug Administration did something it had never done. It cleared the first formal human trials of genetically modified pig kidneys for people with kidney failure. That single decision, paired with months of function in living recipients, shifted xenotransplantation from one-off compassionate procedures to planned clinical medicine. You could feel the field pivot from proof of possibility to proof of process. For a high-stakes milestone like this, it is worth seeing it in black and white: the FDA cleared the first trials. We saw a similar translational inflection in PCSK9’s 2025 editing inflection.
Here is the short version of what changed. Surgeons and bioengineers finally have pigs whose genomes have been edited to remove key immune tripwires, including porcine endogenous retroviruses, and to add human genes that calm rejection. Clinicians are pairing those donor organs with modern immunosuppression playbooks and real-time infection surveillance. And instead of single-hospital exceptions, companies and academic centers are standing up multicenter studies with defined endpoints and safety guardrails. The destination is clear: off-the-shelf kidneys that can be implanted on a timetable, not a waitlist.
What made a pig kidney “human compatible” enough to try
A pig kidney does not work in a human by default. Unedited, it fails almost immediately because the human immune system sees pig sugar molecules on blood vessels as targets. Over the past decade, gene editors and breeders have built donor animals with three broad classes of changes:
- Knockouts that remove the glycan antigens that ignite hyperacute rejection. Think of this as peeling off the flags that human antibodies would otherwise lock onto within minutes.
- Inserted human genes that tune clotting, complement, inflammation, and innate immunity. These are like adding a compatibility layer between two operating systems so they can talk without crashing.
- Inactivation of porcine endogenous retroviruses, or PERVs. These are bits of ancient pig viruses embedded in the pig genome. Shutting them off reduces theoretical infection risk when pig tissues live inside a human body.
These edits do not make a pig kidney identical to a human one. They make it compatible enough that human surgeons can connect pig vessels to human vessels and expect the organ to make urine, clear acid, and regulate electrolytes. Where classic xenografts failed within hours, today’s best-modified kidneys can work for months in people.
From bedside exceptions to multicenter trials
Until now, living-recipient pig kidney implants happened under the Food and Drug Administration’s Expanded Access pathway, often called compassionate use. That pathway lets doctors treat a specific patient with an experimental product when no alternatives exist, while still collecting safety and performance data.
The pivot in 2025 is that companies are moving beyond exceptions. United Therapeutics received clearance for its first kidney trial in February and said it would open with six patients and expand to dozens more as safety allows. In parallel, eGenesis advanced a multi-patient Expanded Access series at Massachusetts General Hospital and later in the year secured study clearance to run a formal trial of its lead kidney product, with a predefined 24-week evaluation window. The details differ, but the architecture is similar: early cohorts are small, eligibility tight, and monitoring intense, with the goal of reaching a multicenter, multiarm study that can support licensure.
Two features of these trials matter for readers who watch the space closely.
- The primary endpoint is near-term and concrete. Centers are focusing on safety, patient survival, graft survival, dialysis independence, and signs of rejection at 24 weeks. The point is to prove that the procedure is not only technically possible but clinically useful on a timeline that investors and regulators can measure.
- The protocols are modular. Immunosuppression backbones can be adjusted across cohorts. That lets teams test whether costimulation blockers, complement inhibitors, or different induction agents change rejection patterns without redesigning the entire study.
A durability line that finally looks like medicine
Durability is not a number, it is a story. In early xenotransplants, we cheered for a few weeks of function. In 2025, a recipient at Massachusetts General Hospital lived with a gene-edited pig kidney for nearly nine months before the organ was electively removed as function declined. That span is not yet a cure, but it is clinical time measured in seasons, not weekends. The case set a new high-water mark and showed rejection can be detected, treated, and sometimes reversed, using familiar transplant tools. For context, see the report on the record nine months in vivo.
Why nine months matters: nephrologists plan care in quarters. A patient who avoids dialysis for most of a year avoids central line infections, hospitalizations, and a measurable hit to quality of life. For regulators and payers, nine months begins to look like functional benefit, not just experimental survival.
The new immunosuppression playbook
Kidney xenotransplantation leans hard on the same logic as human-to-human transplant: prevent the immune system from seeing the graft as an invader, and if it reacts, damp the response fast. The difference is in degree and target.
- Induction is often more assertive, with agents that block costimulation pathways that are particularly important when the graft is from another species.
- Maintenance therapy aims to avoid the brittle edges of over and under suppression. Some programs are layering in complement inhibitors to address the clotting and microvascular injury that crop up in xenografts.
- Surveillance uses modern molecular assays, including cell-free DNA tests and next-generation sequencing, to catch infection and rejection early. Teams are checking for human pathogens and for pig-specific viruses to which the recipient has never been exposed. The diagnostic shift echoes Alzheimer’s blood tests’ 2025 inflection.
A useful mental model is a home security system with more sensors but smarter alerts. You are not just locking the front door. You are watching for early signs of water damage, heat spikes, and motion in unusual places, and you are tuning the system so it does not wake the house every time a cat walks by.
Safety guardrails: infection, ethics, and long follow-up
The scary story everyone worries about is cross-species infection. Porcine endogenous retroviruses are the headline risk, which is why the most advanced donor pigs have those sequences inactivated at the genomic level. That is step one. Step two is a biosecure supply chain: designated pathogen free herds raised in barrier facilities, monitored like biologics plants, not barns. Step three is intensive recipient monitoring with pre-specified protocols for action if a pathogen signal appears.
Ethics guardrails are just as explicit. Trials require robust consent, because recipients agree not only to a transplant but also to years of added surveillance, blood tests, and sometimes restrictions on activities that could increase infection risk. Data and Safety Monitoring Boards assess every serious adverse event. Long-term registries will follow recipients for years, not just months, to track late rejection, chronic viral issues, or unexpected cancer signals.
Equity also has a place in the guardrail conversation. United States dialysis disproportionately burdens lower-income and minority communities. If xenokidneys are approved, enrollment criteria, site selection, and financial support need to ensure the technology does not launch as a boutique product for a narrow zip code. Several trial designs explicitly include patients who are unlikely to receive a human kidney in time, or who are ineligible for human-to-human transplant for medical reasons. That is a practical way to start closing the survival gap while science scales.
Manufacturing and cost: from artisanal to industrial
Unlike human organs, which are recovered and allocated, xenokidneys will be manufactured. That is not a euphemism. It means facilities, standard operating procedures, batch records, quality assurance, and lots that pass or fail. The pig becomes a living bioreactor whose kidneys must meet release criteria before a surgeon ever opens an abdomen.
What it takes to scale:
- Biosecure herds. Donor pigs live in filtered, controlled environments with feed, water, and bedding validated like reagent components. Routine pathogen panels are run on the herd and on every donor.
- Genetic fidelity. The genome edits that make the organs work must be stable. That means careful breeding or cloning strategies with monitoring to avoid drift over generations.
- Just-in-time logistics. A kidney remains viable outside the body for a limited time. Companies will combine cold storage with machine perfusion to stretch that window, so a kidney can travel from a herd in the Southeast to an operating room in Chicago with consistent quality.
- Cost curves that bend with volume. Early units will be expensive. But the inputs are standardizable, and the work lends itself to learning curves. As herds and perfusion platforms scale, per-organ costs should come down, especially compared with the recurring cost of dialysis.
Expect a hybrid business model at first. Hospitals will procure a regulated biologic product rather than match with a donor through an allocation network. Insurers and Medicare will compare the one-time cost of a xenokidney plus follow-up to the repeated annual cost of dialysis, hospitalizations, and complications. If the trials show 12 to 24 months of dialysis-free life with manageable risk, the economic case will look favorable even before the technology reaches peak efficiency.
How the first trials are built
Eligibility is conservative. Early cohorts focus on adults with end-stage kidney disease who are on dialysis, often older than 50, and either ineligible for a conventional human kidney or unlikely to receive one in time. That reduces the ethical tension of trading a known therapy for an experimental one and ensures that the immediate alternative is continued dialysis.
Primary endpoints are pragmatic and centered on 24 weeks after transplant. Investigators will track:
- Patient survival and serious adverse events
- Graft survival and function, including creatinine trends and estimated glomerular filtration rate
- Freedom from dialysis and need for rescue therapies
- Biopsy-proven rejection and microvascular injury
- Infectious events, with special attention to porcine viruses
Secondary endpoints will look further out. Those include 12-month graft function, quality of life metrics, hospitalizations, and the pattern of chronic rejection if it emerges.
Importantly, several programs are designed to grow in place. If the first cohorts meet safety gates, enrollment widens, and more sites come online. That transforms a single-center effort into a multicenter network that can carry the field from feasibility to registrational evidence.
The road to off-the-shelf organs
“Off the shelf” does not mean a pig kidney in a box on a shelf. It means a predictable supply chain and clear criteria that match a given kidney to a patient on a given Tuesday. Here is what has to click to get there:
- Standardized donors. The industry needs one or two well-characterized donor lines with the right edits, raised in compliant facilities, that can be audited like any other biologic supplier.
- Regional perfusion hubs. Expect machine perfusion units to serve as waypoints, extending organ viability and enabling more precise assessment of function before implantation.
- Harmonized protocols. Surgeons in Boston and Houston should follow the same playbook for induction, maintenance, surveillance, and rescue, with only minor site-level variations. That makes outcomes comparable and complications easier to interpret.
- Data infrastructure. Real-time registries will feed safety boards and regulators with common formats for rejection, infection, and lab values. The faster the feedback loop, the faster protocols improve.
Do those things well and a large share of the kidney waitlist becomes addressable with scheduled surgery, not chance.
Infection and ethics guardrails, spelled out
The public will accept this technology only if safety is specific, not just promised. Expect these features to be mandatory before approval:
- Donor herd certification with a clear excluded-pathogen list
- Recipient consent that explains PERV biology in plain language, including what is known and unknown
- Pre-specified response plans for positive viral signals, including contact tracing if needed
- Long-term follow-up commitments that span years and define how data are shared and protected
None of that removes risk. All of it makes risk legible, which is the point of regulation.
What milestones to watch next
- Broader kidney enrollment. Watch for trial updates that show more sites opening and cohorts expanding beyond the first half-dozen patients. Scale is the story.
- First durability readouts. The 24-week marks will land first, followed by 12-month data on dialysis independence, rejection rates, and infections. Those numbers will decide payer coverage.
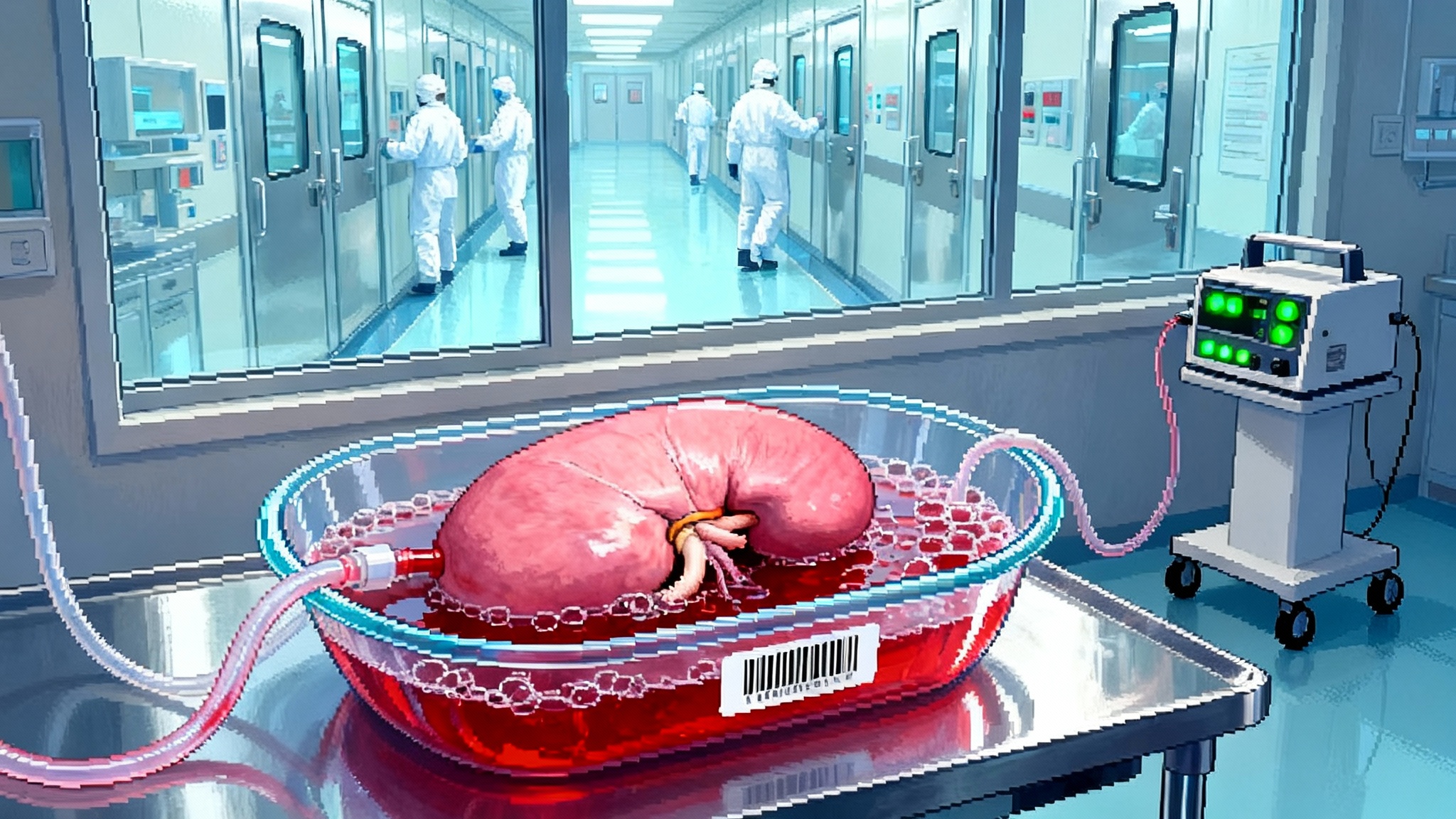
- Liver support trials. A parallel program is starting for acute-on-chronic liver failure that uses a genetically engineered pig liver on an extracorporeal circuit to support humans while the native liver stabilizes or a transplant is found. If it works, it becomes an on-ramp for heart and lung programs that need physiologic support devices as well.
- Manufacturing disclosures. Look for companies to publish herd size, release testing panels, and batch failure rates. That is how you will know whether the cost curve is starting to bend.
- Immunosuppression refinements. Expect protocol updates that add or subtract agents based on biopsy findings and infection patterns, with the goal of minimizing steroid exposure while keeping rejection in check.
The longevity lever hiding in plain sight
Kidney failure shortens lives, often by years. Dialysis keeps people alive, but the five-year survival on dialysis is poor and the day-to-day burden is heavy. A reliable xenokidney would do more than free a person from a chair three times a week. It would cut hospitalizations, reduce infections, and return energy to ordinary days. That is what longevity looks like when you zoom in. It is not a single breakthrough pill. It is a set of systems that remove one heavy weight at a time.
If the first trials prove what the early cases hint, this decade could see organ failure become a tractable mortality problem rather than an unavoidable rationing exercise. The science did not arrive all at once. It arrived the way progress usually does, as a series of better answers to specific, hard questions. In 2025, those answers started moving from the operating room to the clinic. It is the same pattern we saw in plasma exchange human trial insights. Watch carefully. The next data points will not just be headlines. They will be plans for Tuesday surgery.