CHIP Is the New LDL: 2025 Turns Clones Into Treatable Targets
Fresh 2025 evidence moves CHIP from risk marker to modifiable target. ESC data link low-dose colchicine to slower TET2 clone growth, an NIH-backed canakinumab trial is enrolling, CKD ties sharpen risk, and JAMA Cardiology cautions against aspirin for primary prevention.
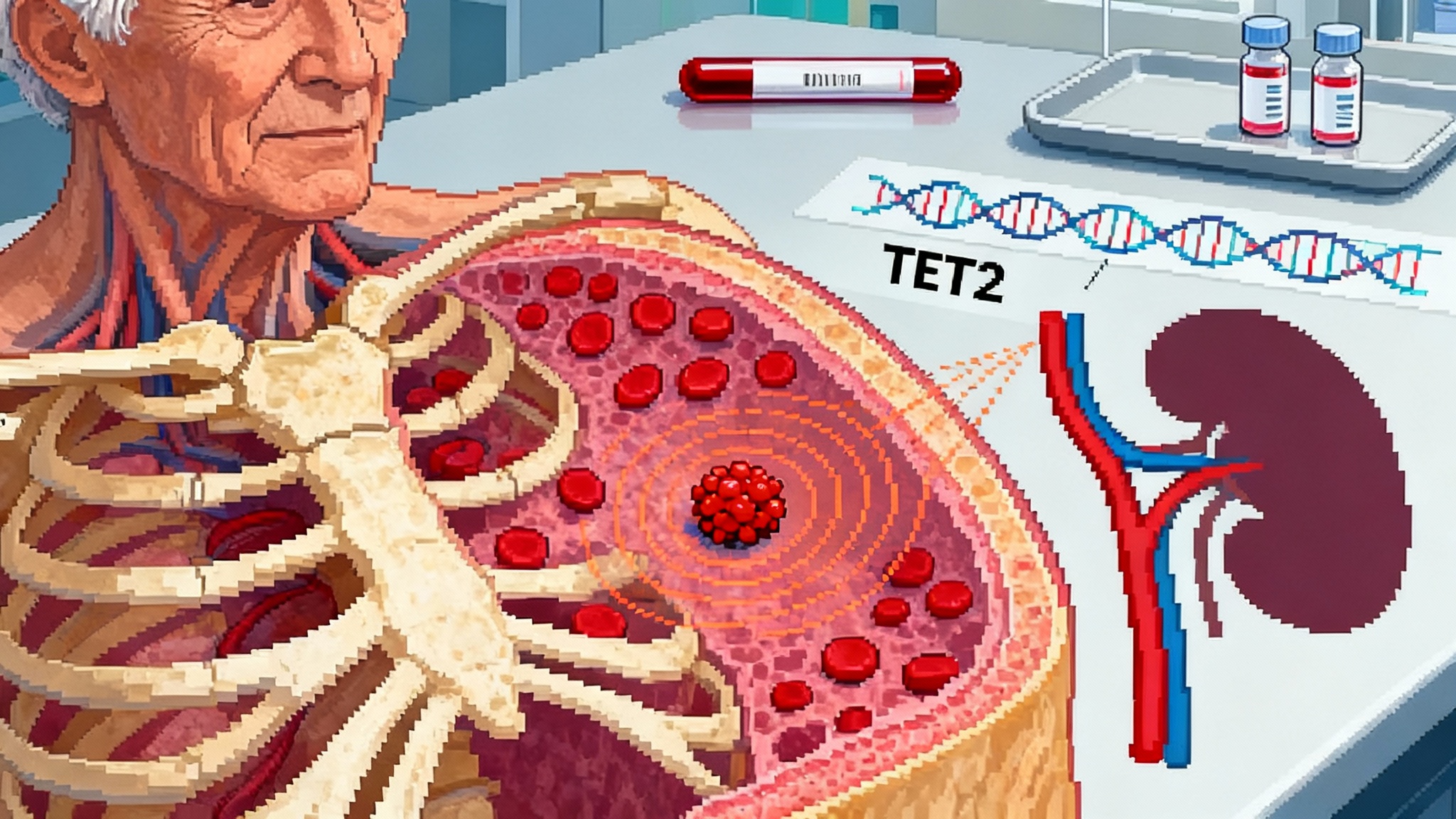
Breaking through: from biomarker to target
For a decade, clonal hematopoiesis of indeterminate potential lived in a medical gray zone. It meant a small fraction of blood-forming stem cells carried cancer-like mutations, but not enough to qualify as blood cancer. Doctors told patients that CHIP tracks with age and higher risk of heart disease and cancer, yet there was little to do except watch. That stance is no longer defensible. In 2025, multiple signals converged to show that CHIP is not just a red flag. It is a target we can modulate.
Start with the headline result from the European Society of Cardiology Congress in August 2025. A prespecified analysis of LoDoCo2 reported that daily low-dose colchicine correlated with slower growth of TET2-mutant clones, the CHIP subtype most tied to cardiovascular risk. The same dataset affirmed that colchicine blunted year-over-year expansion compared with placebo. This is the critical leap from association to modification. It says the clone is not destiny. It is biology that can be nudged. See meeting coverage on the low-dose colchicine slows clonal growth.
If you have followed the CHIP story, the mechanism makes sense. Mutations in TET2 remodel immune responses, amplifying interleukin 1 beta and interleukin 6 signaling. In the artery wall, that translates into more macrophages, more cytokines, and plaques that refuse to quiet down even after LDL is well controlled, including with advances like PCSK9 one-shot LDL editing. Colchicine, a generic microtubule inhibitor used for gout, dampens inflammatory trafficking and inflammasome signaling. The LoDoCo2 cardiovascular benefit was already known; what this ESC analysis adds is a genetic throughline connecting a cheap anti-inflammatory to the growth rate of the specific clones that drive risk.
Direct hit on interleukin 1 beta
There is a second 2025 signal that turns the volume up further. A National Institutes of Health backed, Phase 2 randomized trial sponsored by Massachusetts General Hospital began enrollment on July 1, 2025 to test canakinumab, an interleukin 1 beta neutralizing antibody, directly in people with and without TET2-driven CHIP who also have coronary artery disease and vascular inflammation. The trial will measure change in perivascular fat attenuation by coronary CTA at 48 weeks and track the variant allele fraction of TET2 in blood. Details are posted as the IL-1 beta trial in TET2 CHIP.
Why this matters: in the original CANTOS outcomes study, interleukin 1 beta blockade lowered recurrent events in people with residual inflammation. Post hoc genetic signals hinted that participants with TET2-mutant CHIP saw outsized gains. The 2025 trial is the prospective test of that idea. We are no longer asking if CHIP raises risk. We are asking if an anti-inflammatory can dial down the biology of the clone and the inflammation it fuels, then translate that into fewer heart attacks and less vascular injury. That is interventional geroscience in people, not in mice, building alongside work like the senolytics first real test.
The kidney clue that sharpens risk
Clonal hematopoiesis is often framed as a heart story, yet the kidney data arriving this year push health systems to broaden their view. A four-cohort meta-analysis of more than five thousand individuals with chronic kidney disease found that non-DNMT3A CHIP, especially TET2 variants, associated with substantially higher risk of rapid kidney decline and progression to kidney failure. A complementary mouse model that combined CHIP with induced kidney injury showed lower glomerular filtration rate and kidneys infiltrated with inflammatory cells and fibrosis. The implication is not academic. If non-DNMT3A CHIP accelerates chronic kidney disease, then nephrology clinics are as relevant as cardiology clinics for finding and managing high-risk clones.
Mechanistically, this tracks with what we have learned. DNMT3A-only mutations often behave as a longevity marker with limited systemic impact, particularly at low clone sizes. In contrast, TET2, ASXL1, and JAK2 skew hematopoietic stem cells toward myeloid lineages primed for inflammatory signaling. Kidneys become collateral damage in a body whose innate immune system idles high.
Aspirin is not the workaround
Aspirin once seemed like a tidy fix for an inflammatory disease of the artery. A 2025 analysis in JAMA Cardiology argues otherwise in older adults without established cardiovascular disease. In that prespecified substudy of a large randomized trial, CHIP was linked to more clinically significant bleeding but not to more primary prevention cardiovascular events, and there was no evidence that low-dose aspirin provided a differential benefit for those with CHIP. Coupled with modern guidance that already restricts aspirin in primary prevention because of bleeding, the take-home is clear. Aspirin is not the CHIP therapy. If anything, it may add harm without offsetting benefit in many older carriers. Clinicians should not reach for aspirin as a generic answer to an inflammatory clone.
Make CHIP screening practical: a risk-stratified playbook
We are poised to make CHIP as routine as LDL in risk workups, but only if screening is targeted and the follow-on steps are clear. Here is a pragmatic framework any large health system or clinic can implement in the next 12 months.
Who to screen first
- Adults 60 and older with one of the following: a history of coronary artery disease, heart failure with preserved or reduced ejection fraction, chronic kidney disease stages 2 to 4, or a persistent high-sensitivity C-reactive protein above 2 mg/L despite guideline LDL targets.
- Patients in cardio-oncology pathways: before anthracycline chemotherapy, chest radiation, Bruton tyrosine kinase inhibitors, or tyrosine kinase inhibitors for chronic myeloid leukemia. Oncology sequencing already surfaces CHIP as an incidental finding. Use that information rather than letting it die in the report footer.
- Adults of any age with premature atherosclerosis in the family, recurrent plaque inflammation on imaging despite optimal lipids, or current smokers with additional inflammatory comorbidities such as psoriasis or rheumatoid arthritis.
How to test
- Use a blood-based next generation sequencing panel that includes the canonical drivers DNMT3A, TET2, ASXL1, JAK2, and common spliceosome genes such as SRSF2. Ask the lab to report variant allele fraction down to 2 percent and to flag likely germline variants for separate counseling.
- Pair the test with basic labs that matter for downstream decisions: high-sensitivity C-reactive protein, complete blood count, lipid panel, lipoprotein(a), estimated glomerular filtration rate, and urine albumin-to-creatinine ratio.
- Build a consent and counseling script. Patients should know this is not a cancer diagnosis and that a small fraction of carriers develop blood malignancy, typically at higher clone sizes or with multiple hits. The near-term focus is cardiovascular and kidney risk.
How to interpret and act
- No CHIP detected at 2 percent variant allele fraction or higher: follow standard prevention, repeat testing in 3 to 5 years if clinical risk rises.
- DNMT3A-only CHIP at 2 to 10 percent variant allele fraction without systemic inflammation: focus on risk factor intensity. Aggressively treat blood pressure and LDL cholesterol, ensure vaccinations, and track the clone annually for growth. DNMT3A-only at small clone size is often a passenger rather than a driver.
- TET2, ASXL1, JAK2, spliceosome genes, any clone at or above 10 percent variant allele fraction, or multiple CHIP drivers: escalate. Bring cardiology or cardio-nephrology into the loop. Consider coronary CT angiography if the patient’s risk level and symptoms justify it, and intensify lipid lowering including one-dose siRNA for Lp(a) if elevated. For patients with coronary disease already, discuss low-dose colchicine given the genetic signal in TET2-mutant clones and the established atherosclerosis benefit in prior outcomes trials.
- Rapidly expanding clone: if the variant allele fraction rises by more than 5 percentage points over one year or doubles over two years, repeat counts and peripheral smear, and consider early hematology input to rule out evolving myeloid disease.
Document and close the loop
- Add a discrete CHIP field in the electronic record with gene, variant allele fraction, date of test, and a simple risk tier. Set reminders for annual clone checks in higher risk tiers.
- In cardio-oncology clinics, turn incidental CHIP from tumor sequencing into an automatic referral to cardio-oncology or preventive cardiology with a prefilled order set for labs and counseling.
- Build a registry. Health systems do this for familial hypercholesterolemia and aortic aneurysm. CHIP registries will let you track clone trajectories, adverse events, and therapy responses across thousands of patients.
Therapies to use now, test next, and build toward
Here is a staged approach that aligns with what we know today and what is on deck for the next three years.
- Use now in the right patient: low-dose colchicine 0.5 milligram daily in patients with established coronary disease who also carry TET2-mutant CHIP, barring drug interactions or intolerance. The genetic signal at ESC 2025 suggests clone-level benefit, and the cardiovascular risk reduction was already demonstrated in earlier trials. Monitor for diarrhea and drug interactions, especially with strong P-glycoprotein inhibitors.
- Consider in trials or specialty centers: interleukin 1 beta blockade for people with TET2-driven CHIP and vascular inflammation, given the trial now enrolling. The dosing in the current study is quarterly, which makes adherence realistic for older adults with polypharmacy. The therapy is expensive and immunosuppressive, so trial infrastructure matters.
- Explore next: downstream anti-inflammatory targets such as interleukin 6 receptor blockade or NLRP3 inflammasome inhibitors may be logical for non-TET2 high-risk clones. These warrant prospective studies with clone size and inflammatory imaging readouts.
- Keep on the shelf: aspirin for primary prevention in CHIP carriers without established disease. The 2025 analysis does not show a CHIP-specific benefit and highlights bleeding risk in older adults. Post-procedure antiplatelet decisions still follow coronary stent and guideline pathways.
Why this is accelerationist geroscience
Geroscience often gets stuck between fascinating mechanisms and distant outcomes. CHIP breaks that stalemate. The biology is clean enough to track in blood. The inflammatory machinery is druggable. The clinical events, from heart attack to kidney failure, are common and costly. Feedback loops can be measured quarterly rather than yearly. This is not a moonshot that requires new molecular platforms. It is a stack we already own: a short list of gene assays, routine labs, simple imaging, two to three anti-inflammatory options, and a care team that knows how to follow a clone the way it follows LDL or hemoglobin A1c.
Think of CHIP as a spark in the bone marrow that throws embers into the endothelium and the kidney. LDL lowering removes the fuel, but the embers keep landing. Anti-inflammatory therapy is the screen that stops them. Clone-modulating therapy, like colchicine for TET2, is the fan that weakens the spark at its source. The future is to combine both screens and fans, calibrated by genotype and clone size.
What could go wrong, and how to prevent it
- Overdiagnosis without a plan: screening drives anxiety if clinicians do not know what to do next. The fix is a protocolized pathway with clear thresholds and clinic ownership, plus shared decision-making scripts so patients understand the purpose and limits of testing.
- Overtreatment with immunosuppression: potent anti-inflammatory agents raise infection risk and cost. That is why risk stratification by gene, clone size, and inflammatory biomarkers is essential. Pair therapy with vaccinations and infection vigilance.
- Fragmented data: oncology labs often detect CHIP incidentally, but the result gets buried in a PDF. Demand discrete data fields from lab partners and build automated referral rules in the electronic record.
- Equity gaps: sequencing access and follow-up care can skew to well-resourced clinics. Health systems should subsidize testing for high-risk groups, integrate screening into routine nephrology and cardiology visits for older adults, and use remote monitoring for clone tracking where visits are a barrier.
The bottom line
In 2000, the cholesterol debate turned when statins changed hard outcomes. In 2025, CHIP is going through the same pivot. ESC data connect a generic anti-inflammatory to slower growth of riskier clones. An NIH-backed trial is testing whether targeted interleukin 1 beta blockade can cool arteries and shrink clone fractions in people with TET2-driven CHIP. Kidney studies push action beyond the heart. JAMA Cardiology reminds us that aspirin is not the answer in primary prevention. The result is a playbook any health system can run now.
Screen older adults and cardio-oncology patients who have the most to gain. Read the gene and the clone size, not just the presence of CHIP. Use anti-inflammatory therapy where it is proven and enroll patients in trials that aim at the core pathway when risk is highest. Build registries so we learn faster. That is how CHIP becomes the new LDL in practice, not in metaphor, and how interventional geroscience moves from promise to routine care.