Teeth That Grow Back: TRG-035 Starts First Human Trials
Japan has moved tooth regeneration from lab promise to the clinic. Toregem BioPharma’s anti-USAG-1 antibody, TRG-035, has orphan status and is now in first-in-human testing, opening a new path beyond dentures and implants.
A quiet revolution in the dentist's chair
Japan just turned a long-running lab dream into a real clinical program. Toregem BioPharma's anti-USAG-1 antibody, code-named TRG-035, was designated an orphan drug by the Ministry of Health, Labour and Welfare on September 29, 2025 for severe congenital oligodontia, a rare condition in which many teeth never form. That recognition matters because it signals official support and paves a faster regulatory road for a first-in-class therapy that aims to regenerate a natural tooth, not replace it with a prosthetic. The company's announcement confirms that TRG-035 is now on that path, with Japan treating it as a priority for a small but underserved group of patients (Toregem orphan designation).
This story began moving from lab bench to hospital in 2024, when a Japanese research team received clearance to begin the first investigator-initiated trial in people. That first-in-human study is being conducted with Kyoto University Hospital and Kitano Hospital, and it focuses initially on safety and early signs of biological activity in adults who are missing teeth. The launch of clinical testing is more than a milestone for dentistry. It is a test case for targeted organ regeneration, similar in ambition to the clinical turn in xenotransplantation described in our 2025 xenotransplant pivot.
The biology in one sentence and one metaphor
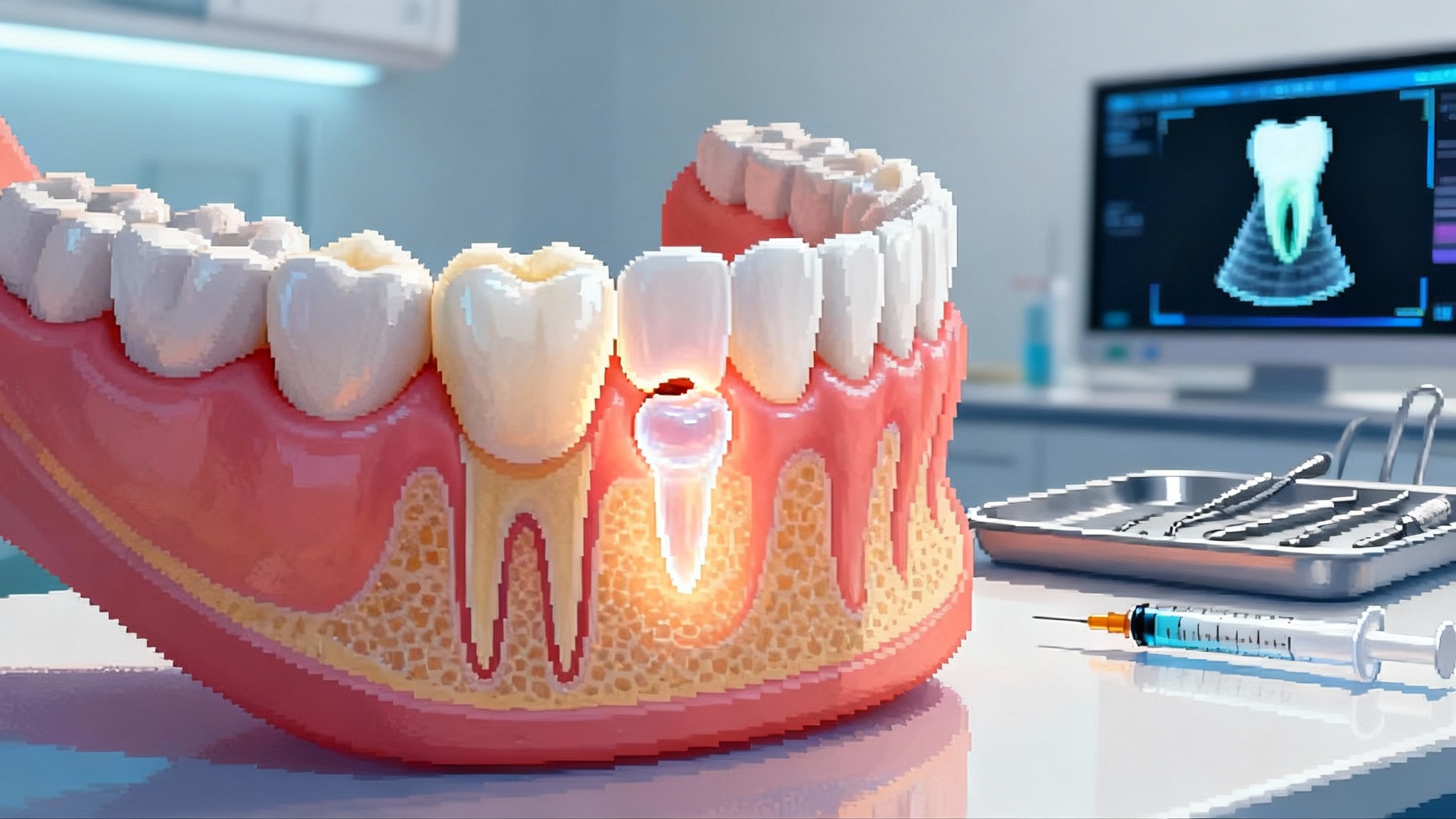
One sentence: USAG-1 is a protein that acts like a brake on the bone morphogenetic protein and Wnt signaling pathways that shape teeth during development; TRG-035 is a monoclonal antibody that lifts that brake so a tooth germ can form and mature.
One metaphor: Imagine tooth development as a construction site with two foremen, Bone Morphogenetic Protein and Wnt. They coordinate when, where, and how a tooth takes shape. USAG-1 is the overly cautious safety inspector who keeps shutting the site down. TRG-035 politely but firmly escorts that inspector off the premises for a limited time. With the stop orders lifted, the crew can complete a tooth that should have been there in the first place.
In animal models, blocking USAG-1 was enough to restart the program. Researchers first showed in mice that a single dose could tip the balance toward a new, properly patterned tooth. They then moved to ferrets, which, like humans, develop two sets of teeth. In ferrets the approach again produced a natural tooth that erupted and occluded. Those results built the case that the biology is conserved across species, and that a temporary push on these pathways can be sufficient to set the process in motion.
Why this could extend healthspan, not just fix a smile
Regenerating a tooth is not a vanity project. Chewing efficiency is a physical function with direct consequences for aging. Older adults who can chew well tend to eat more fibrous vegetables, nuts, and protein. Better mastication supports higher protein intake, which protects lean mass, reduces fall risk, and helps maintain independence. Replacing a missing molar with a natural, rooted tooth also stabilizes the bite, preserves alveolar bone, and improves speech and social confidence. For a broader look at how restoration of function can influence aging trajectories, see our review of the 2025 plasma exchange trial.
Partial dentures and implants help, but they are not perfect substitutes for a living organ. Dentures can slip and reduce bite force. Implants do not have a periodontal ligament, so they transmit force differently and can lead to bone remodeling problems in some patients. By contrast, a regenerated tooth can, in principle, reconnect with local tissue and nerves, respond to pressure, and maintain local bone through natural micro-motion.
From lab promise to human protocols
The first clinical study in Japan starts with adults who are missing at least one tooth. The early objective is safety. Investigators will check whether a single or short series of doses is tolerated, and they will monitor blood markers, imaging, and any changes in nearby tissue. Because the goal is to form a new tooth germ and allow it to mature, readouts are slower than for a typical pain or infection drug. X-ray and cone-beam computed tomography can reveal early mineralized structures. Dentists will also watch for eruption, occlusion, pulp vitality, and signs of a functioning periodontal ligament.
Why adults first, when the first approved use is likely to be a pediatric rare disease? Adult volunteers are often easier to recruit and consent for first-in-human trials, and adult anatomy allows more precise imaging. If the adult study shows acceptable safety and biological activity, the program will move toward children with congenital oligodontia, where the medical need is highest and the potential benefit is life-long. Japan's regulators placed the first stepping stone in March 2024, when the Pharmaceuticals and Medical Devices Agency approved an investigator-led plan for a trial of a tooth regeneration drug for congenital edentulism in collaboration with Kyoto University Hospital (Kitano Hospital trial notice). For context on parallel clinical frontiers in sensory restoration, compare the momentum described in 2025 gene therapy restored hearing.
The safety questions the trial must answer
Every regenerative therapy has a precision problem: you want the effect in exactly the right tissue, at the right time, and nowhere else. For TRG-035, the specific risks to watch are straightforward and manageable, but they are not trivial.
- Mispatterned teeth: If the signal persists too long or acts in the wrong place, you could see a supernumerary tooth that is misshapen or misaligned. That would likely require extraction or orthodontic management. Trial protocols should include scheduled imaging to catch early mispatterning, along with predefined criteria for intervention.
- Ectopic teeth: There is a theoretical risk that a tooth bud could form slightly off target, for example near the maxillary sinus. Careful local delivery and dosing windows are designed to minimize this, and imaging is intended to detect it early.
- Off-target bone effects: Because Bone Morphogenetic Protein influences bone formation, researchers will watch for abnormal calcifications in surrounding tissue. Short exposure and controlled dosing aim to avoid chronic pathway activation.
- Immunogenicity: TRG-035 is a monoclonal antibody. As with any biologic, the team will test for anti-drug antibodies and infusion reactions, and monitor laboratory parameters.
These are the same categories of risks that oral and maxillofacial surgeons already manage with other interventions, which is why trial design includes integrated dental and medical oversight.
The regulatory path and why orphan status matters
Orphan designation in Japan recognizes that a disease affects a small population and that there is a strong rationale and development plan. The designation brings tangible benefits that can speed development. Those benefits typically include priority review, fee reductions, and a long reexamination period after approval during which similar products are not approved, which functions like a market exclusivity window of about ten years. For a niche medicine such as TRG-035, that window increases the likelihood that investors will fund expensive manufacturing, quality testing, and pediatric studies.
What might the timeline look like if everything goes right? After the adult safety readout, the program would pivot to studies in children with severe congenital oligodontia. Pediatric studies would likely use careful dose finding, longer follow-up, and validated developmental endpoints. If efficacy and safety are shown in this rare group, the sponsor could seek an initial approval in Japan, possibly before broader studies in acquired tooth loss.
The competition is not standing still
Regenerative dentistry is a portfolio of technologies rather than a single race. TRG-035 addresses the question of whole-tooth formation. Other approaches aim at different pieces of the puzzle:
- Bioengineered tooth germs: Japanese groups have shown that transplanting a lab-grown tooth germ in mice can produce a fully functional tooth with proper structure, nerve responses, and occlusion. Translating that to humans would require dependable sources of the two cell types that make a tooth and a reliable way to integrate the organ with the jaw. This approach is elegant but logistically complex, and it would likely start as a surgical procedure in specialized centers.
- Periodontal and bone regeneration: Surgeons already use biologics to coax periodontal tissues and bone to regrow around compromised teeth. These tools can stabilize existing teeth and improve implant sites but do not create a new tooth.
- Pulp regeneration: Endodontic techniques are evolving from inert fillings toward biologically active scaffolds that can preserve or restore living pulp in immature teeth. This improves tooth vitality but does not address missing teeth.
- Enamel regeneration: Self-assembling peptides are being used to rebuild enamel mineral in early lesions. These products help preserve teeth before cavities progress but are not designed to replace a whole tooth.
- Dental implants and prosthetics: Modern implants and high-precision dentures continue to improve and will remain essential, especially for patients who are poor candidates for a biologic or who need rapid restoration.
Think of the field as a toolbox. TRG-035 would add an entirely new tool rather than replace the others. In the near term, clinicians will likely combine parts of these strategies to rebuild function case by case.
What to watch from 2026 to 2030
- Adult safety and activity readout in Japan: Expect initial results on tolerability, dosing, and early biological signals. Look for objective imaging of a tooth germ and evidence of healthy eruption pathways.
- Pediatric initiation in congenital oligodontia: The first pediatric subjects will test whether the biology translates where the need is greatest. Parents and clinicians will be watching for practical outcomes such as improved bite force, speech, and nutrition.
- Dosing, delivery, and localization: Early work has used intravenous dosing. Developers will explore local injections or controlled release near the target site to sharpen the effect and reduce systemic exposure.
- Patterning and orthodontics: Even a well-formed new tooth may need guidance. Expect protocols that integrate orthodontic planning, including space maintenance, eruption guidance, and occlusion management.
- Manufacturing and quality: Monoclonal antibodies must meet rigorous quality standards. Watch for updates on scaled production, stability data, and release testing. These steps often determine how quickly a therapy can reach more hospitals.
- First regulatory filing in Japan: If pediatric data are strong, an initial filing for severe congenital oligodontia could come before the end of the decade. Timelines depend on data robustness and safety signals.
- Expansion studies in acquired tooth loss: The biologic logic suggests potential in adults who lost teeth to decay, gum disease, or trauma. Trials here will need larger numbers and longer follow-up to prove consistent patterning and function in mature jaws.
- International trials: After initial Japanese studies, look for partnerships or trials in Europe and the United States. Health systems will pay close attention to health economic analyses that compare long-term function and costs against implants and dentures.
Practical implications for dentists and patients right now
- Documentation and imaging: Clinics that might participate in studies should standardize cone-beam computed tomography and digital impression workflows. Consistent imaging makes patterning and eruption easier to assess.
- Patient selection and counseling: Identify candidates with congenital oligodontia or with single-tooth deficits that are stable and well documented. Discuss the novelty of the approach, the possibility of mispatterning, and the need for follow-up.
- Nutrition and rehabilitation: If a new tooth forms, chewing capacity will change over months, not days. Plan for staged dietary adjustments and occlusion checks rather than a one-time fix.
- Orthodontic readiness: Coordinate early with orthodontists. Eruption guidance and space maintenance can turn a promising biological result into a stable, functional outcome.
The bottom line
TRG-035's progress marks a shift in how we think about teeth. For a century dentistry excelled at repair and replacement. A targeted biologic that convinces the mouth to build a new tooth asks the body to do the work it was designed to do. Japan's orphan-drug nod and first-in-human study move that idea out of science fiction and into the clinic. If the next five years bring clear evidence of safe, well-patterned teeth that erupt, occlude, and feel, then rebuilding a bite may someday start with a prescription rather than a drill.