Longevity
Longevity
Articles under the Longevity category.
CHIP Goes Mainstream: Slowing Aging Clones to Protect Hearts
{"excerpt":"Fresh 2025 data suggest low-dose colchicine can slow growth of TET2-driven clonal hematopoiesis while new studies link CHIP to heart failure and mortality. The case for adding inexpensive CHIP genotyping to routine cardiometabolic care is now strong."}
The CMV Vaccine That Could Rewire How We Age in 2025
Moderna's pivotal cytomegalovirus vaccine readout in 2025 could do more than protect babies. If it works, it may slow immune aging, improve responses to other shots, and open a path to longevity vaccines against latent viruses.
Engineered Tregs After the Nobel: Immunity Rejuvenated
October 2025 put regulatory T cells in the spotlight and turned immune rejuvenation from concept to clinical plan. Engineered Treg therapies and thymus regeneration are moving toward trials aimed at infections, inflammaging, and better vaccine responses.
One-Shot PCSK9 Editing Could Recode Heart Disease Risk
In 2025, first-in-human data, Fast Track status, and a major pharma deal pushed one-time PCSK9 base editing from theory to clinical reality. Here is how a single dose could compress lifetime LDL exposure and what must still go right.
Pig Kidneys Enter Trials: The 2025 Xenotransplant Pivot
In 2025, gene-edited pig kidneys moved from heroic one-offs to formal clinical trials. With multi month, dialysis free living at Mass General and FDA clearances for the first xenokidney trials, the field is now chasing measurable gains in late life health.
Aging Biomarkers After LDT Rollback: The Trial-Grade Playbook
After the FDA’s September 2025 shift on lab-developed tests, epigenetic clocks and proteomic age panels have a clear path from CLIA labs to clinical trials and payer adoption. This playbook shows how to build trial-ready endpoints, win coverage, and deploy usable tools in primary care.
Epigenome Editing Enters Humans, Longevity’s Next Switch
Between January and April 2025, epigenome editing crossed into human trials. Tune Therapeutics secured approvals in Hong Kong and New Zealand and raised $175 million to launch TUNE-401 for chronic hepatitis B. Here is why this pivot could reshape prevention-first longevity.
FDA clears path for first canine anti-aging pill in 2025
The FDA’s 2025 signal to Loyal and new funding for the Dog Aging Project could turn dogs into the fastest proving ground for longevity drugs. Here is how a pet-first strategy can validate biomarkers, de-risk mechanisms, and reshape human trials by 2028.
Switching Off TTR: CRISPR’s One-shot Plan Against Amyloid
Intellia’s in vivo CRISPR therapy aims to permanently switch off transthyretin (TTR), the protein that seeds ATTR amyloid in heart and nerves. With Phase 3 trials underway, a single infusion could shift care from chronic dosing to prevention if safety and outcomes hold.
Midlife Inflection: The Vascular-First Longevity Playbook
A 2025 multi-organ proteomics atlas reveals a midlife inflection and puts vascular health at center stage. This practical playbook gives readers in their 40s and 50s measurable steps, endpoints, and tools to add healthy years.
Three-parent IVF ignites a new era in mitochondria medicine
Peer-reviewed results from Newcastle University’s mitochondrial donation program show eight births with minimal mutant mtDNA after pronuclear transfer. Here is how this 2025 proof point reframes adult mitochondria therapies and policy.
Turning Plasma Exchange Hype Into Testable Human Biology
A rigorous 2025 randomized trial reported multi-omics shifts and aging-clock improvements after therapeutic plasma exchange, especially with IVIG. Here is what that signal means for safety, cadence, costs, and how clinics can test it without hype.
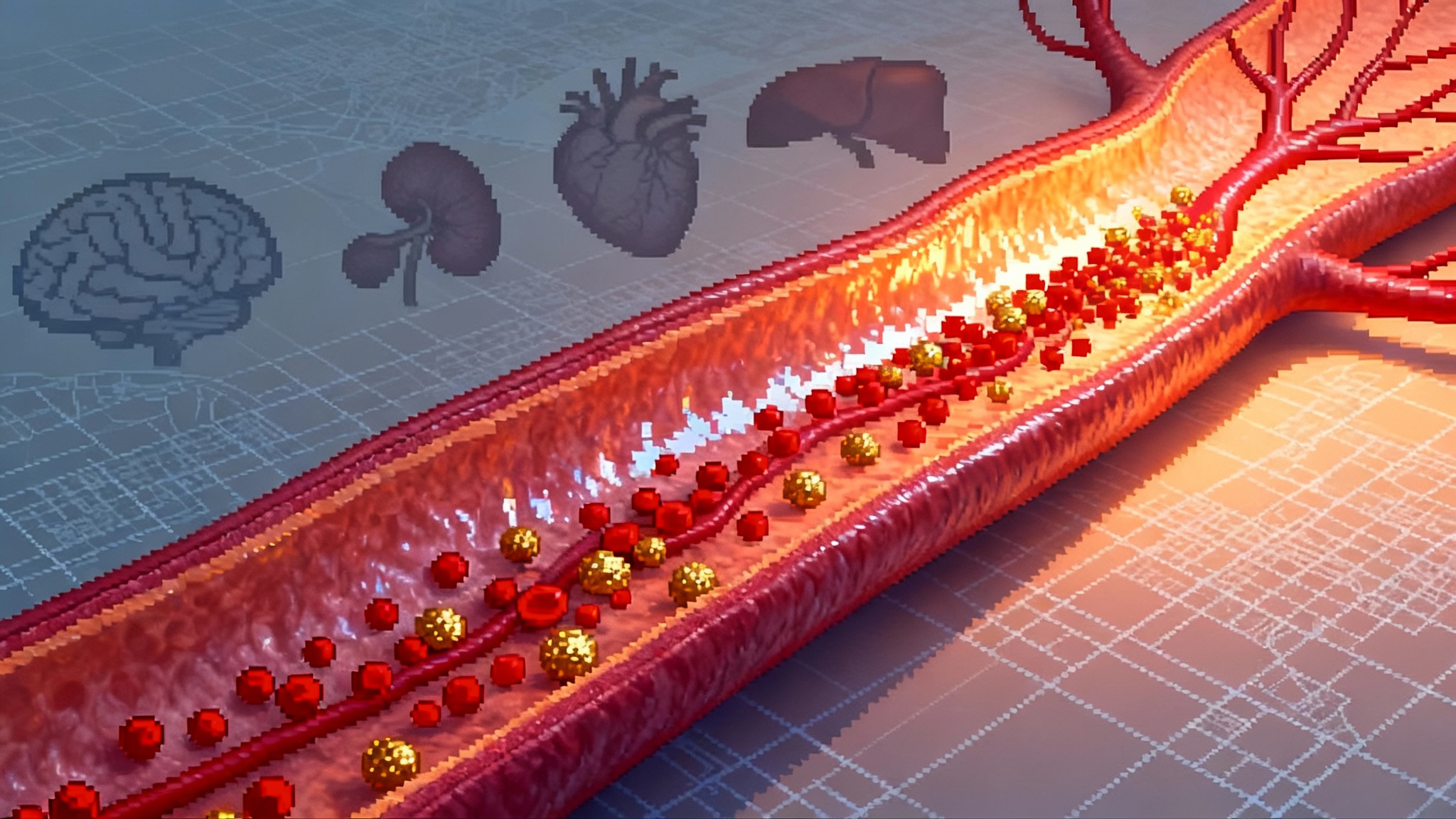
The Next Longevity Lever: Lp(a) Suppression Goes Mainstream
In 2025, lipoprotein(a) finally became actionable. Durable RNA silencers cut Lp(a) 80 to 95 percent and an oral blocker emerged, setting up twice-yearly injections or a daily pill as a new backbone for cardiovascular prevention.
CMV’s 2025 readout could launch longevity vaccines
Moderna’s cytomegalovirus vaccine is nearing a pivotal 2025 efficacy readout that could recast prevention as a lever on immune aging. Here is what matters for regulators, payers, and anyone tracking the first real test of longevity vaccines.
ESC 2025 makes CHIP clinical: slowing aging blood clones
Fresh ESC 2025 data suggest that daily low-dose colchicine slows the growth of TET2-driven clonal hematopoiesis, an age-linked blood clone tied to cardiovascular and cancer risk. Here is a practical case for midlife CHIP screening and a near-term path to clone-targeted therapies.
The Thymus Revival: Naive T Cells as Longevity's Edge
The overlooked thymus is taking center stage. In 2025, fresh data, a pediatric approval, and government funding converged to make naive T cell restoration a credible strategy to harden immunity, improve vaccine responses, and extend healthy years.
From Eye to Brain: OSK Reprogramming Enters Trials
On September 25, 2025, FDA INTERACT feedback gave YouthBio a path to clinic for brain-targeted partial reprogramming. Life Biosciences is moving ER-100 toward first-in-human vision trials, making ophthalmology the launchpad for longevity tech.
One-time LDL Editing Goes Mainstream after Lilly-Verve Deal
Eli Lilly’s 2025 acquisition of Verve and the first Phase 1b PCSK9 base editing data mark the moment when one-and-done LDL cholesterol reduction moved from moonshot to clinical reality. Here is what it means and what to watch next.