Switching Off TTR: CRISPR’s One-shot Plan Against Amyloid
Intellia’s in vivo CRISPR therapy aims to permanently switch off transthyretin (TTR), the protein that seeds ATTR amyloid in heart and nerves. With Phase 3 trials underway, a single infusion could shift care from chronic dosing to prevention if safety and outcomes hold.
The news, and why it matters
A single infusion that turns off an amyloid-forming protein at its source is no longer a thought experiment. Intellia’s in vivo CRISPR therapy for transthyretin amyloidosis is deep into late-stage testing. The cardiomyopathy study began dosing in 2024, and the companion polyneuropathy study started in April 2025. Together they test whether one edit in the liver can durably lower transthyretin and change the arc of a classic age-associated disease. The cardiomyopathy trial, called MAGNITUDE, dosed its first patient in March 2024, confirming that Phase 3 is in motion and global in scope, as noted in MAGNITUDE first patient dosed in March 2024.
Why does this matter beyond one condition? Transthyretin amyloidosis is an archetype for protein misfolding with age. Wild-type transthyretin, and inherited variants like V122I, can misfold into rigid fibers that infiltrate the heart and nerves. If shutting down transthyretin in the liver proves safe and lasting, the field would have a template for converting other chronic proteinopathies from lifelong management to one-and-done biology, echoing the momentum behind one-time LDL editing goes mainstream.
The problem, plain and simple
Transthyretin’s day job is to ferry retinol and thyroxine. With age or mutation, a fraction of the protein comes apart and re-assembles into amyloid. In the heart, that stiffens the ventricle, raises filling pressures, and leads to heart failure symptoms. In peripheral nerves, the same deposits injure axons and small fibers, causing pain, numbness, and autonomic problems.
In the United States, cardiomyopathy from wild-type transthyretin is increasingly detected as imaging and awareness improve. Polyneuropathy is more common in hereditary disease. Both are progressive. Left untreated, cardiomyopathy raises mortality and hospitalization, while neuropathy erodes independence.
Current drugs attack the problem from two angles. Stabilizers such as tafamidis or acoramidis bind transthyretin tetramers so they do not fall apart. Silencers such as small interfering RNA or antisense oligonucleotides reduce production in the liver. These have changed outcomes, especially for neuropathy, yet they require chronic dosing and constant payer negotiation. In March 2025 the United States Food and Drug Administration broadened Alnylam’s RNA interference drug, vutrisiran, to include cardiomyopathy, a win that signals how valuable transthyretin lowering has become in the clinic, per FDA expansion of Amvuttra to ATTR-CM.
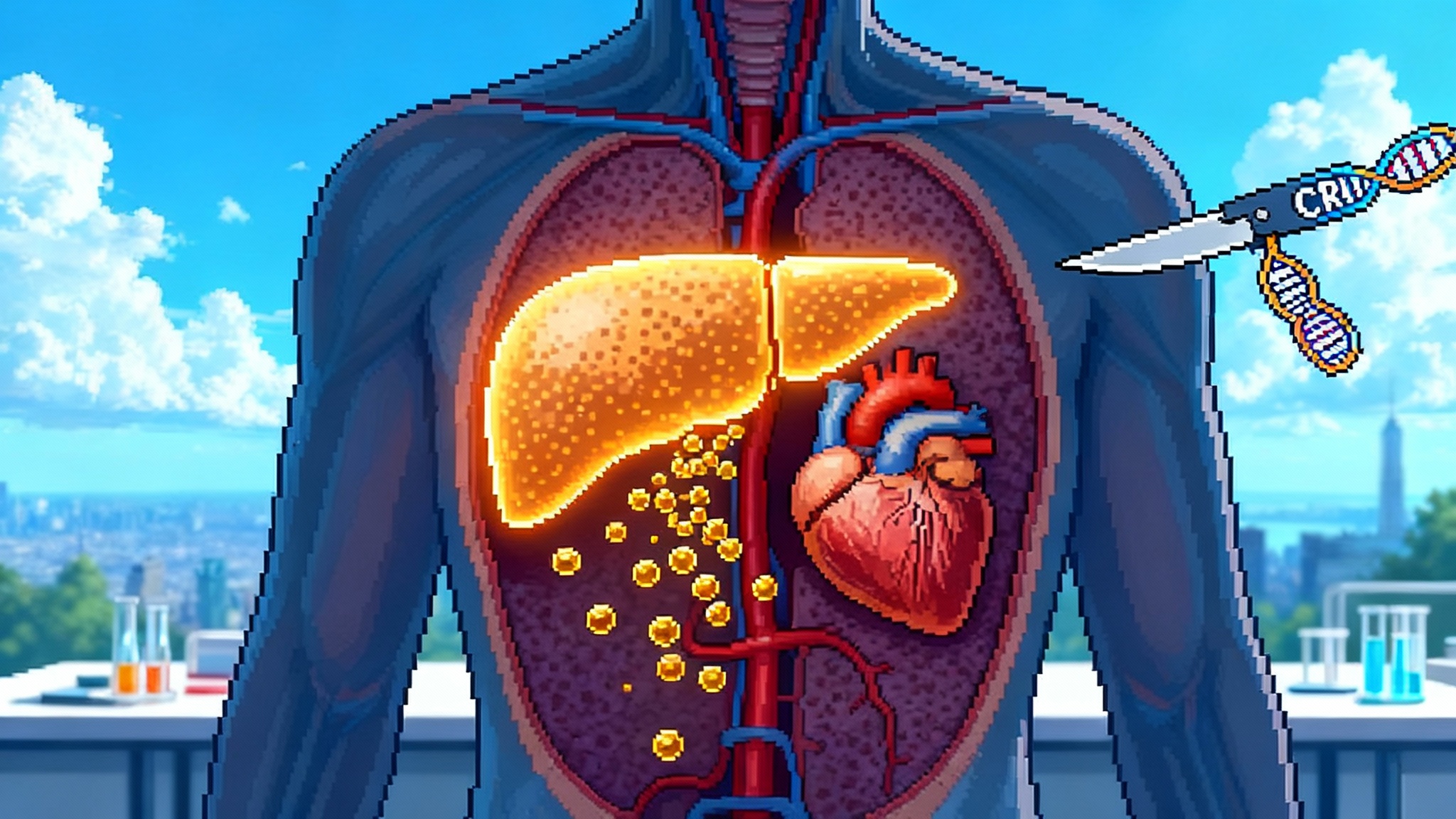
CRISPR proposes a different bet. If a single edit cuts the gene in hepatocytes, circulating transthyretin drops for years, possibly for life. No refills, no adherence drift, and a straight line from mechanism to disease biology.
How the edit works
Intellia’s candidate, now called nexiguran ziclumeran and formerly NTLA-2001, uses a lipid nanoparticle to deliver two payloads to the liver. One is messenger RNA that cells translate into the Cas9 enzyme. The other is a guide RNA that steers Cas9 to the TTR gene. Cas9 makes a cut, the cell repairs the break with small insertions or deletions, and the gene is disrupted. Less messenger RNA means less protein, and the level of circulating transthyretin falls.
The liver is a natural first target for in vivo editing. Hepatocytes are easy to reach with nanoparticles, they divide slowly in adults, and the blood is a short commute from any protein they make. Early clinical data suggest transthyretin can be driven down by roughly ninety percent or more after a single dose, and that reductions remain stable for years in the small number of patients followed long term. Phase 3 must now show that deep lowering translates into fewer hospitalizations, better function, and survival benefits at a scale regulators and payers trust.
The safety signals to watch
Editing is not the same as dosing a traditional drug, so the risk ledger looks different. Three categories will draw particular scrutiny.
-
Liver stress. The therapy acts in hepatocytes. Transient elevations in alanine transaminase or aspartate transaminase have been observed in earlier studies of in vivo CRISPR and are a key monitoring point in Phase 3. Regulators will pay attention to the timing, the severity, and whether enzyme spikes correlate with deeper on-target editing or with infusion reactions.
-
On-target biology. Transthyretin carries retinol. If you lower it to near zero, patients need vitamin A supplementation and ophthalmologic monitoring. Night vision issues, dry eyes, or other signs of hypovitaminosis A must be taken seriously, especially in older adults who may have marginal nutrition. Transthyretin also binds thyroxine, although thyroid hormones are carried largely by other proteins, so routine thyroid function tests will still be wise in the first years after editing.
-
Off-target editing and genomic integrity. Modern guide design, high-fidelity Cas9 variants, and in silico plus in vitro off-target screens have raised confidence, but Phase 3 is the first large-scale look at tens of thousands of edited livers over time. Safety labs will track clonal liver enzyme signals, and investigators will follow for rare events like benign nodular hyperplasia or other idiosyncratic findings. Because the payload is transient messenger RNA, ongoing Cas9 expression is not expected. Even so, immune responses to Cas9 and to the lipid nanoparticles themselves require vigilance, premedication protocols, and clear stopping rules.
One more nuance matters for patients with eye involvement. Some transthyretin is produced in the retinal pigment epithelium and in the choroid plexus. Liver editing likely cannot touch those pools. That means ocular amyloid can be an independent course that needs its own monitoring strategy.
From chronic care to one-and-done prevention
If a single edit can durably silence transthyretin, the entire care model for this disease shifts. Consider the current state for cardiomyopathy. Stabilizers reduce mortality and hospitalizations, but patients stay on therapy indefinitely. Silencers reduce transthyretin more directly, yet they remain maintenance care, administered every month or quarter. Gene editing reframes the problem as a one-time intervention that could be offered earlier, potentially before major organ damage anchors a poor prognosis. That mindset fits a vascular-first longevity playbook.
That opens a prevention mindset. Carriers of the V122I variant, which is common in people of West African ancestry, could be identified through routine genetic testing and offered editing before symptomatic cardiomyopathy. In wild-type disease, risk stratification is trickier. We do not have a single blood test that says a 65-year-old with concentric left ventricular hypertrophy is on the cusp of amyloid deposition. Imaging biomarkers and proteomic signatures will have to get sharper if prevention is the prize, much as cardiometabolic genetics are becoming actionable in Lp(a) suppression goes mainstream.
Regulators will ask for endpoints that matter to patients. For cardiomyopathy, expect composite outcomes that combine death and cardiovascular hospitalization, judged over several years. For polyneuropathy, validated functional scales, gait speed, and patient-reported outcomes sit alongside serum transthyretin as mechanistic anchors. Deep lowering is a compelling biomarker, but clinically meaningful change will carry the approval.
The payer calculus, in plain numbers
Payers have spent years absorbing the cost of chronic ATTR therapy. Tafamidis carries a United States list price in the high two hundreds of thousands of dollars per year. Vutrisiran’s quarterly injections add up to several hundred thousand dollars annually at list prices. Even with discounts and patient assistance, the present value over five to ten years reaches numbers that make actuaries sweat.
A one-time edit will not be cheap. Gene therapies for other diseases have launched between one million and three million dollars. If an ATTR edit lands in that range, the comparison becomes a question of durability and avoided events. Assume a two million dollar one-time cost that removes the need for five to eight years of high-cost therapy, prevents multiple heart failure admissions, and keeps a patient working and independent. In that scenario, total costs can converge in only a few years, with the gene edit pulling ahead as the curve stretches out.
Real contracts will likely include guardrails. Outcomes-based rebates that trigger if transthyretin does not fall by a predefined percentage. Installment payments over three to five years that stop if the patient loses coverage or if prespecified clinical milestones are not met. Centers of excellence that manage infusion, vitamin A supplementation, and long-term follow-up in a single program to reduce waste and variation.
Payers will also care where the bill lands. A hospital outpatient infusion may fall under a medical benefit, like Medicare Part B, which makes utilization management different than a pharmacy benefit under Part D. Employers may push for reinsurer carve-outs to smooth the rare but large claims. State Medicaid programs will ask for federal sharing and special financing, since ATTR skews older but not exclusively Medicare.
Finally, expanded approvals for chronic silencers in cardiomyopathy, like the March 2025 decision for vutrisiran, set a market reference that gene editing will have to match on value. If an edit can beat quarterly dosing on both outcomes and lifetime cost, the budget math starts to work for everyone.
Who goes first if it works
Assuming Phase 3 delivers, triage will be key in the early years.
- Symptomatic cardiomyopathy with early or mid-stage disease. Patients who are sick enough to benefit, but not yet too frail to tolerate an infusion and transient liver stress, will line up first. Those already on stabilizers or silencers could be transitioned after a washout period.
- Hereditary polyneuropathy with rapid progression. Polyneuropathy trials are smaller, but the biology is similar. Patients with hereditary variants and measurable functional decline, despite standard therapy, will be clear candidates.
- Pre-symptomatic carriers. This is the prevention frontier. Offering a permanent edit to someone who feels fine raises fresh ethics and reimbursement issues. Expect expert-center sign-off, genetic counseling, and a shared decision process that documents the person’s risk, values, and alternative options.
The road to other misfolding targets
If silencing transthyretin proves both safe and effective, where else could the blueprint travel?
- Fibrinogen A alpha chain amyloidosis. The amyloid-forming protein is made in the liver, so a similar editing strategy is plausible. Kidney involvement dominates, so endpoints and specialty oversight would be different, but the delivery vehicle and editing logic could be reused.
- Apolipoprotein A-I variants. Some hereditary apoA-I mutations form amyloid with hepatic production. Partial silencing or allele-specific editing might work, yet cholesterol transport biology places a premium on sparing normal function. That argues for base editing or allele-selective approaches rather than a blunt knockout.
- Light chain amyloidosis is another story. The culprit protein comes from a plasma cell clone, not the liver, so the right tool remains myeloma therapy rather than hepatocyte editing.
- Central nervous system proteopathies, like beta-amyloid in Alzheimer’s disease, are enticing but technically distant. Editing in the brain needs better delivery, tighter control, and a much higher safety bar.
The pattern is clear. When the offending protein is secreted by the liver, and when partial or full suppression is tolerated, in vivo editing is in play. When the protein comes from a tumor clone, a non-hepatic tissue, or has non-redundant normal roles, the strategy needs new tricks.
Manufacturing, access, and real-world follow-up
A one-time therapy still has a supply chain. Lipid nanoparticles and clinical-grade messenger RNA demand specialized manufacturing, quality control that can catch subtle impurities, and cold chain logistics. Health systems must stand up infusion capacity and lab pipelines to measure transthyretin and vitamin A at the right intervals.
Post-marketing follow-up should be designed into the program from day one. A registry that tracks liver labs, vitamin A levels, eye symptoms, hospitalizations, and survival will give payers and regulators confidence. It also helps physicians and patients calibrate expectations. If deep lowering correlates with specific thresholds of benefit, dose selection and monitoring can become more precise.
What to watch next
- First, the shape of the Phase 3 readouts. Look for early pharmacodynamic snapshots of transthyretin reduction, followed by interim clinical events. Cardiomyopathy studies often hinge on composite endpoints; seeing consistent movement across hospitalization, exercise capacity, and quality of life will be persuasive.
- Second, the safety table. Regulators will comb for liver signals, infusion reactions, and any hint of off-target effects. The absence of late surprises will matter almost as much as early efficacy.
- Third, payer pilots. Expect the first real contracts to set patterns for outcomes-based rebates and installments. The design of these agreements will influence the speed and breadth of adoption.
- Fourth, competition from chronic drugs that are improving. Stabilizers and silencers are not standing still. If chronic therapy outcomes keep improving, the edit must clear a higher bar on both value and convenience.
The bottom line
Turning off transthyretin at the source is a bold idea that happens to match the disease biology. Amyloid builds because the protein keeps coming. If the faucet is shut, the sink can finally drain. Phase 3 will tell us if that metaphor holds in real hearts and nerves. If it does, one-and-done care would not just simplify life for patients and families; it would recast how we invest in healthspan, with prevention delivered as a precise edit rather than a lifelong drip.
The agenda from here is straightforward. Prove that deep lowering leads to lasting clinical benefit without new safety liabilities. Build the contracts that let payers say yes without breaking budgets. Design the registries that keep learning alive after approval. Do those things, and ATTR could become the first age-linked proteopathy where modern medicine edits the problem upstream, then lets the body do the rest.