One-time LDL Editing Goes Mainstream after Lilly-Verve Deal
Eli Lilly’s 2025 acquisition of Verve and the first Phase 1b PCSK9 base editing data mark the moment when one-and-done LDL cholesterol reduction moved from moonshot to clinical reality. Here is what it means and what to watch next.
The inflection point arrives
On July 25, 2025 Eli Lilly closed its acquisition of Verve Therapeutics, planting a pharma giant’s flag in one-and-done cardiovascular prevention and signaling that permanent low-density lipoprotein reduction is no longer science fiction but a strategic priority. The company framed the deal as a path to lifelong risk reduction with a single treatment, not another drug to refill forever. That is a striking statement for heart disease, still the leading global killer. Lilly completes acquisition of Verve Therapeutics.
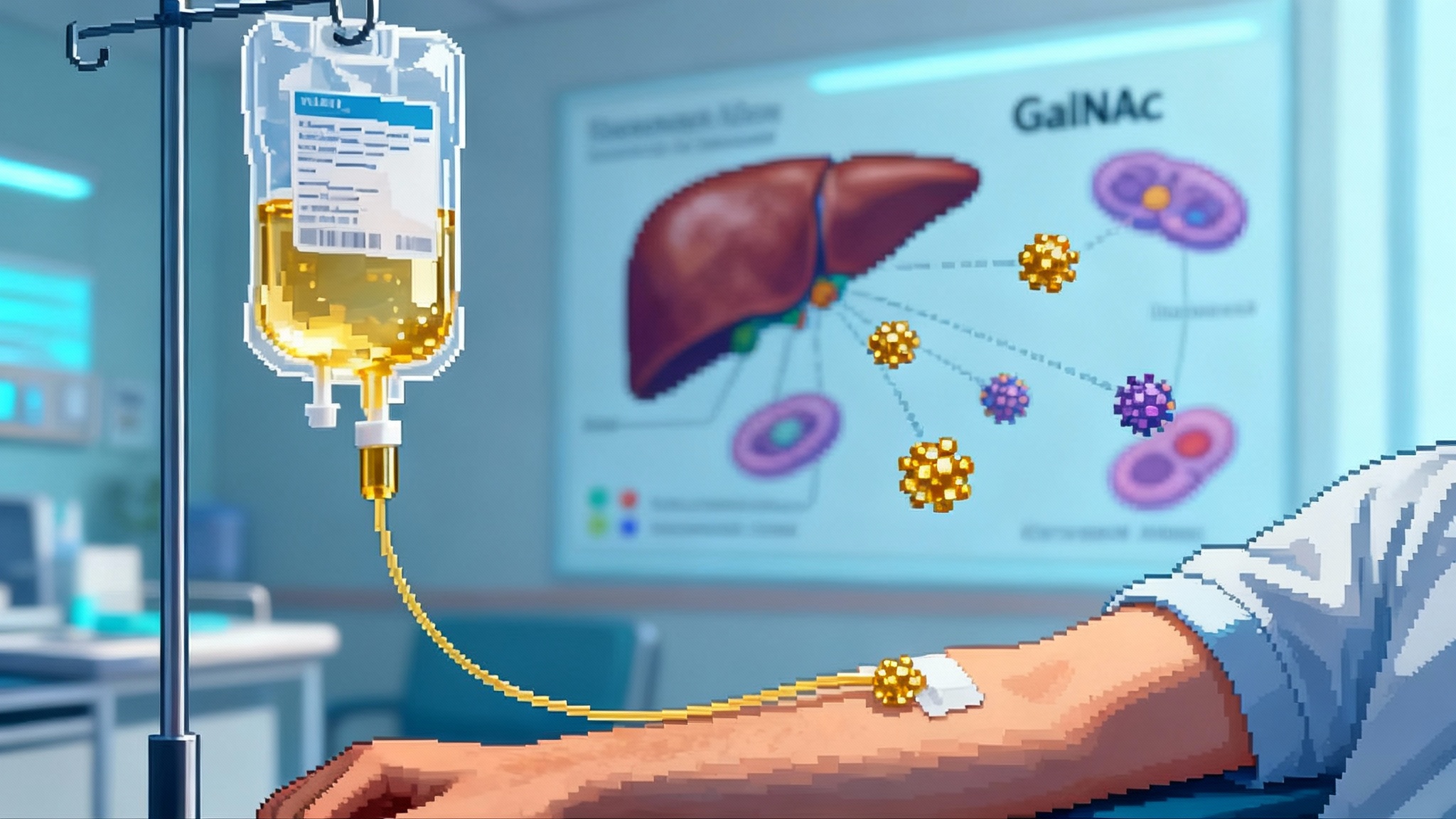
Two months earlier, we saw the data that made this believable beyond boardrooms. Verve’s Heart-2 Phase 1b readout of VERVE-102, an in vivo adenine base editing approach to silence PCSK9 in the liver, reported dose dependent lipid effects after one infusion, with mean low-density lipoprotein reductions of roughly 53 percent at the 0.6 mg/kg cohort and a maximum reduction near 69 percent, alongside a clean early safety profile. That is the sound of a new category entering the clinic, not just the lab. positive Heart-2 Phase 1b data.
This is the news hook. The larger story is what permanent low-density lipoprotein lowering could do to compress lifetime cardiovascular risk, how it compares with statins, PCSK9 antibodies and inclisiran, and the real questions investors, clinicians, and payers should track from here. For context on other one-and-done modalities moving into the clinic, see the in vivo CAR-T playbook and the first-in-human trial shift.
What a one-and-done LDL edit actually does
Think of the liver as a sorting facility that pulls low-density lipoproteins out of circulation using LDL receptors. PCSK9 is like a mischievous manager that forces those receptors into the trash compactor. Shut down PCSK9 in liver cells and more receptors survive, so the liver clears far more cholesterol particles from the blood.
VERVE-102 delivers an adenine base editor and a guide RNA to hepatocytes in a lipid nanoparticle. The edit introduces a tiny spelling change in PCSK9 that turns the gene off. The editor does its work and is cleared; the change remains in the cell’s DNA. No implanted device, no refill schedule, no ongoing adherence. The promise is durable physiology, not chronic pharmacology.
If you prefer a simpler picture: imagine the area under the curve of lifetime low-density lipoprotein exposure as the stack of boxes that the liver must remove to prevent artery plaque. Statins and injectables help workers move boxes faster. Editing reduces how many boxes show up each day. The cumulative workload over decades falls dramatically.
A clear way to think about risk compression
Randomized trials show that lowering low-density lipoprotein later in life cuts annual major cardiovascular events by about one fifth per 39 mg/dL drop. Genetic studies suggest that when you live with lower levels from early in life, the benefit per unit drop roughly doubles because plaque never gets the same head start. A one-time edit in midlife will not recreate a childhood of clean arteries, but it moves prevention earlier on the timeline than any adherence dependent therapy can realistically achieve.
Two scenarios make this concrete:
- A 48 year old with heterozygous familial hypercholesterolemia has an on treatment low-density lipoprotein of 140 mg/dL despite a high intensity statin. A one time edit that reliably lowers low-density lipoprotein by 50 to 60 percent could bring that to the 50 to 70 mg/dL range. Over the next decade, that reduction could translate into a double digit absolute risk reduction for heart attack and revascularization compared with staying at 140 mg/dL, especially if started before a first event.
- A 62 year old with prior stents and an on treatment low-density lipoprotein of 85 mg/dL on statin plus ezetimibe might accept a one time procedure to push levels into the 30s or 40s with no future adherence burden. In secondary prevention where annual event rates are higher, even a modest additional relative reduction can yield meaningful absolute risk avoided.
The big idea is that a permanent drop compresses lifetime exposure. If a single treatment halves low-density lipoprotein for the remainder of someone’s natural lifespan, the cumulative plaque building area under the curve falls far more than any intermittent therapy that is stopped, forgotten, or not covered.
How it stacks up against the incumbents
Let’s put the main options on the same field and focus on concrete tradeoffs.
- Statins: Inexpensive generics, oral, and proven to reduce heart attacks and strokes. Typical low density lipoprotein reductions are 30 to 50 percent, with the highest intensity regimens at the top of that range. The two issues are adherence and ceiling. Roughly half of patients stop statins over time, and for high risk or genetically driven hypercholesterolemia, even the best statin often cannot get patients to very low targets on its own.
- PCSK9 monoclonal antibodies (evolocumab, alirocumab): Potent, reliable 50 to 60 percent reductions in low-density lipoprotein and event reduction demonstrated in large outcomes trials. Injections every 2 to 4 weeks and specialty pharmacy logistics make adherence harder than a daily pill. List prices have come down, yet payers still gate access and persistence can wane over years.
- Inclisiran: Given twice yearly by a clinician after the initial loading schedule, so adherence is largely system driven. Low density lipoprotein reductions of about 50 percent are common in practice. U.S. list price per dose is in the mid three thousand dollar range and the Food and Drug Administration recently allowed monotherapy use, extending its reach. This option turns cholesterol management into a calendar item rather than a lifestyle commitment.
- Oral PCSK9 inhibitors in development: Merck’s oral agent has shown up to about 60 percent low-density lipoprotein lowering over eight weeks in Phase 2 with Phase 3 programs underway. If approved, a daily pill that delivers antibody like potency will expand access dramatically, though adherence and long term cost still matter in real life.
- One time base editing of PCSK9: Early human data now show mean low-density lipoprotein reductions above 50 percent at active doses after a single infusion. That is within the potency band of antibodies and inclisiran, without repeat dosing. The open questions are durability beyond the first year, consistency across populations, extremely rare risks that only appear in large exposures, and the price and coverage model for a permanent intervention.
A short way to think about it: statins are the foundation, antibodies and inclisiran are the power tools for patients who need deeper lowering or cannot tolerate statins, oral PCSK9 pills may soon offer powerful convenience, and editing is the architecture change that could make the entire house more stable with one build.
What to watch next
Durability
- Signal to watch: the slope of low-density lipoprotein and PCSK9 protein over 12 to 24 months in Phase 1b and Phase 2 cohorts, and the fraction of hepatocytes carrying the edit. Flat lines a year out suggest true durability; upward drift would argue for partial editing or biological compensation.
- Practical marker: if a single infusion keeps low-density lipoprotein suppressed by roughly 50 percent or more for at least two years without retreatment, durability becomes a tailwind for both payers and guideline writers.
Off-target and long term safety
- The mechanism matters. Base editors do not cut both DNA strands, which may reduce some risks, but they can in theory cause off-target base changes in DNA or RNA. Delivery via lipid nanoparticles transiently exposes the liver to the editor, which helps, yet rare events only appear when thousands are treated and followed for years.
- What to look for: comprehensive genome wide off target assessments, careful measurement of liver enzymes and platelets, and the pattern of any serious adverse events relative to cardiovascular baseline risk. Expect regulators to require long term follow up measured in years, not months, with particular attention to cancer surveillance and pregnancy guidance.
Population level impact
- A simple forecast: in secondary prevention cohorts, annual major adverse cardiovascular event rates can hover around a few percent. A durable relative risk reduction of 20 to 30 percent translates into absolute reductions of roughly 0.6 to 1.0 percent per year. Treat one million such patients once, and you are plausibly preventing many thousands of heart attacks and revascularizations over a decade. The earlier you intervene in the risk timeline, the larger the cumulative benefit.
- System upside: fewer recurrent events free up cath labs, reduce repeat procedures, and ease clinician bandwidth. The downside is that health systems must stand up infusion capacity, longitudinal registries, and genetic counseling at scale. For a look at how systems adapt to frontier therapies, see the pig kidney trial precedent.
Payer and regulatory path
- Surrogate endpoints: the Food and Drug Administration has a long history of accepting low-density lipoprotein reduction as a basis for approval while requiring post marketing outcomes data. For permanent genome editing, expect a higher safety bar and mandated long term follow up. The likely regulatory sequence is early approval in high risk populations where unmet need is clear, then expansion as evidence grows. Related policy shifts around validation and surveillance echo themes in FDA’s LDT reversal.
- Payment models: a one time therapy will invite outcomes based contracts and annuity style payments. A practical reference point is the cumulative cost of many years of PCSK9 therapy versus a single infusion. If editing is priced near a decade of specialty therapy and demonstrates durable effect, actuaries can model long term savings. If durability is uncertain, payers will push for warranties, step edits through statins and pills, and tight patient selection.
Follow ons beyond PCSK9
- Lipoprotein(a): elevated Lp(a) is an independent risk driver. Verve has disclosed a program to edit the LPA gene. If safe and effective, a one time Lp(a) edit combined with PCSK9 editing could deliver a two axis risk reduction that no chronic therapy matches today.
- ANGPTL3: turning off this gene reduces multiple atherogenic lipoproteins, not just low-density lipoprotein. An ANGPTL3 edit could serve patients with refractory hypercholesterolemia or mixed dyslipidemia. Watch for early human data and any signal of liver fat changes, given the pathway’s role in lipid handling.
Timelines that matter
- 2025 to 2026: more complete Phase 1b and early Phase 2 data for PCSK9 editing, including the first real durability curves. Expect expanded geographies and more diverse cohorts. Safety databases still small, so interpret outliers carefully.
- 2026 to 2028: pivotal designs come into view. Oral PCSK9 agents may reach approval in this window, setting a new standard for potent, convenient low-density lipoprotein lowering. Editing will need to show why permanence wins on a risk benefit basis compared with a cheap, strong, widely accessible pill.
- 2028 and beyond: outcomes data, registry scale evidence, and the first multi target editing strategies. If Lp(a) and ANGPTL3 follow ons hit clinical milestones, the field shifts from single knob to control panel.
Practical implications today
For clinicians
- Start thinking in phenotypes and trajectories, not just today’s lab value. Who benefits most from a permanent intervention: severe genetic hypercholesterolemia, very premature coronary disease, patients with recurrent events who cannot adhere to or tolerate chronic therapy.
- Make documentation airtight. Genetic diagnoses, prior drug trials, and adherence history will become the basis for payer approval in early years.
For patients
- Expect a careful discussion of permanency. You cannot turn an edit off, so the consent process will focus on unknowns as much as benefits. The procedure is an infusion over hours, then monitoring. If you respond, you may never need another cholesterol drug. If you do not, you still have all the standard options.
For policy makers and payers
- Build the rails now. Set up outcomes based contracts that pay over time, create registries to capture rare events, and plan for equitable access that does not require a concierge clinic. The earlier health systems prepare, the faster we can translate trial data into fewer heart attacks.
An accelerationist case, grounded in tradeoffs
Lilly’s purchase of Verve and the Heart-2 data shifted permanent low-density lipoprotein lowering from an intriguing prototype to a pipeline with sponsors, timelines, and global infrastructure. The accelerationist view is simple: if a safe, one time infusion can cut a lifetime of cardiovascular risk, then getting it to appropriate patients sooner will save lives and money. The grounded view adds conditions: we need year over year durability, unambiguous off target surveillance, and a payment model that rewards prevention without breaking budgets. Those conditions are testable, and they are being tested now. If the curves stay flat, the safety profiles stay boring, and the payer math clears, one and done low-density lipoprotein editing will not just join the toolkit, it will change the expectations for what prevention can do.