Pig Kidneys Enter Trials: 2025’s Biggest Longevity Bet
For the first time, U.S. regulators have authorized formal studies of gene-edited pig kidneys. Here is what changes for patients, safety, and longevity if xenotransplantation moves from one-off surgeries to scalable care.
The year pig kidneys walked into the clinic
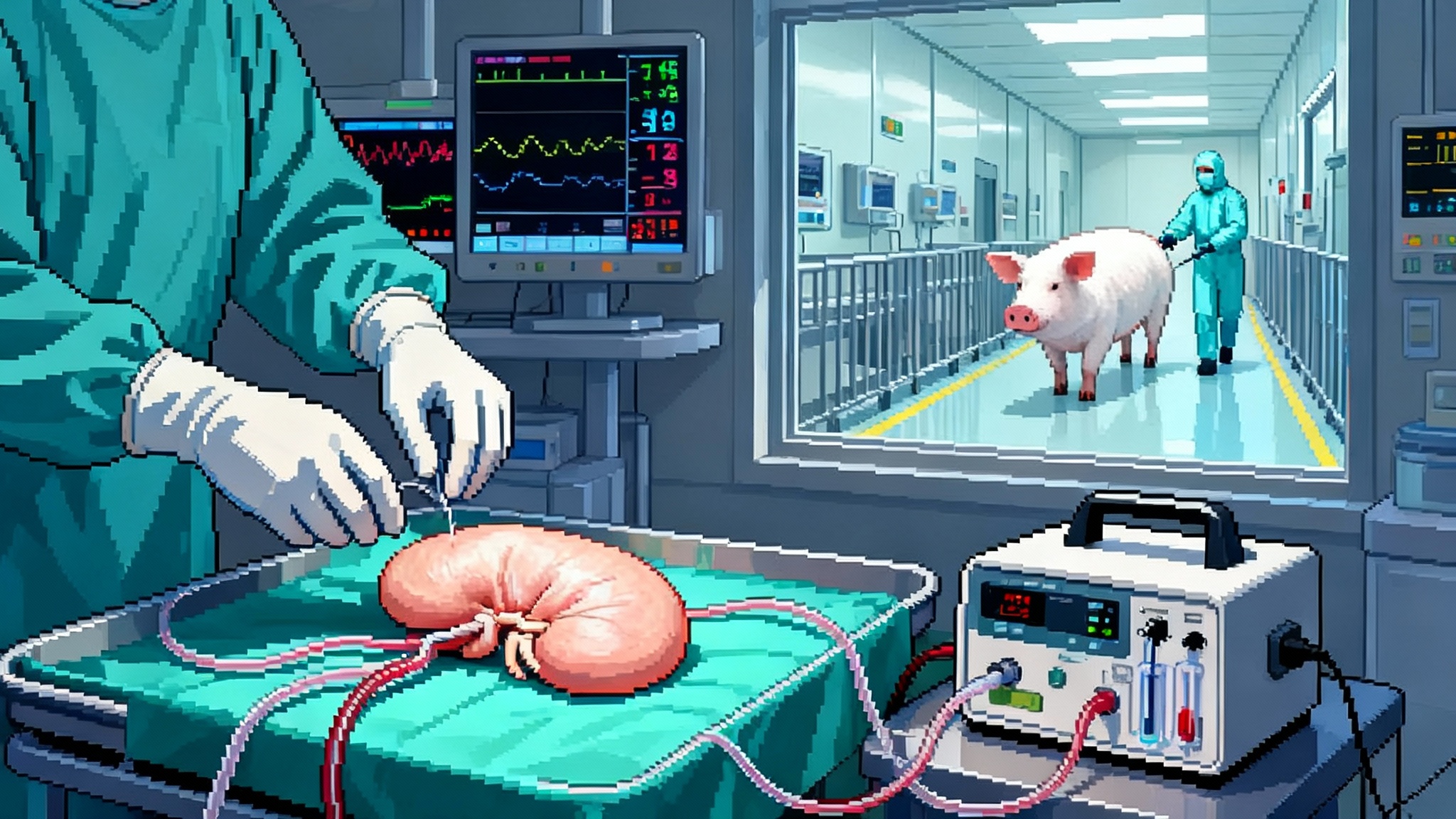
In early February 2025, federal regulators cleared two companies to begin transplanting gene-edited pig kidneys into patients as part of formal clinical studies. United Therapeutics and eGenesis will start with small cohorts and expand if safety and function look strong. This is not a speculative headline. It is the moment xenotransplantation left the lab and entered the ward. The decision matters because it creates a path from compassionate one-offs to standardized medicine, with trial endpoints, manufacturing plans, and a real regulatory finish line. For the first time, kidney failure has a plausible new supply chain.
The headline claim deserves a primary source. See the report that the United States Food and Drug Administration gave the first greenlights to clinical studies and outlined initial enrollment plans for both firms: FDA clears first xenokidney trials.
The approvals did not appear in a vacuum. Surgeons had already built a bridge of evidence using the Food and Drug Administration’s expanded access pathway. Massachusetts General Hospital transplanted an eGenesis kidney into a living patient in March 2024, then followed with additional compassionate-use cases, including a surgery in late January 2025. NYU Langone Health used United Therapeutics organs in two high-profile 2024 cases, including a transplant that paired a heart pump with a pig kidney in a patient too sick for a traditional transplant. These cases showed surgical feasibility, early function, and key safety lessons that shaped trial designs.
Why xenokidneys matter for longevity
Kidney failure is one of medicine’s silent mortality engines. Dialysis can keep people alive, but it is a time tax with a heavy toll on the heart, blood vessels, and immune system. Mortality on long-term dialysis is several times higher than in the general population. Transplanted kidneys restore the continuous filtration that human physiology expects, and that consistently delivers longer survival than dialysis. The problem is supply. Tens of thousands of people wait each year while only a fraction receive a kidney. A scalable, safe, and predictable source of organs shifts this math. If xenokidneys can keep patients off dialysis for meaningful stretches of time, the effect on all-cause mortality is immediate and concrete.
Compared with pharmacologic gains like GLP-1s and a mortality reset, organ replacement directly removes a major mortality driver. Think of organ failure like a blocked highway at rush hour. Dialysis is sending a tow truck to each stranded car every few days. A working kidney is clearing the blockage so traffic flows. Xenokidneys aim to add new lanes. If those lanes are reliable, the traffic jam of waitlists shrinks and the downstream crashes decline.
What is actually inside a gene-edited pig kidney
A xenokidney is not a wild-type pig organ. It is a made-for-human device built from living tissue.
- The starting point is immunology. Humans mount fierce antibody and cellular responses to certain sugar patterns on pig cells. Modern source pigs remove the three main culprits by disabling the genes GGTA1, CMAH, and B4GALNT2. This lowers the risk of hyperacute rejection, which can destroy an organ within minutes.
- Compatibility is also about clotting and inflammation. To make the vascular interface behave more like human tissue, companies add human genes that regulate complement and coagulation, such as CD46, CD55, thrombomodulin, or endothelial protein C receptor. The exact transgene set differs by firm. The goal is a calmer blood contact surface and fewer microthrombi in the graft.
- Size control matters. Some pigs include a growth hormone receptor edit so the organ does not outgrow its human host.
- Endogenous viruses are addressed at the blueprint level. Porcine endogenous retroviruses are stitched into pig genomes. eGenesis uses clustered regularly interspaced short palindromic repeats to disable dozens of these sites and then layers on the immunology edits noted above. United Therapeutics takes a different path with fewer edits plus a clever immunology assist described next.
These edits are not ornamental. They target the specific failure modes that ended early xenograft attempts: explosive complement activation, coagulation chaos, antibody attack, and the theoretical risk of latent pig viruses waking up in human cells.
Teaching tolerance: immunomodulation and the thymus assist
Even with gene edits, a pig kidney is foreign. You still need immunomodulation to keep the immune system in a cooperative mood. Two approaches matter right now.
- Modern drug protocols rely on combinations of induction antibodies, calcineurin inhibitors like tacrolimus, antiproliferative agents like mycophenolate, and steroids. Some teams add costimulation blockade that interferes with the handshake T cells need to activate.
- A second, bolder strategy tries to retrain the recipient’s immune system to see the organ as self. United Therapeutics has advanced a thymus co-transplant concept that places a bit of the pig’s thymic tissue with the kidney. In the thymus, developing T cells are educated to tolerate the host. Placing the pig thymus under the kidney’s capsule creates a tutorial classroom on site. The first combined mechanical heart pump and gene-edited pig kidney with thymus was performed in April 2024 at NYU Langone Health, and it showed early function without obvious rejection. Read the center’s description of the approach in thymus-augmented pig kidney transplant.
Why does this matter for real-world longevity? If thymic co-transplantation reduces the intensity or duration of immunosuppression, it lowers infection risk and drug toxicity while stabilizing graft health. That is the path to durable function in older and more fragile patients.
The hard safety questions, asked plainly
Safety is the hinge that will decide whether xenokidneys scale.
- Rejection phenotypes. Clinicians will watch for hyperacute rejection in the first hours, acute antibody-mediated rejection over days to weeks, and chronic rejection over months. Biopsies will be scored with established criteria and paired with biomarker panels. The edits aim to blunt these phases, but the field must prove that histology matches hope.
- Coagulation and microangiopathy. When pig endothelium meets human blood, subtle incompatibilities can trigger thrombotic microangiopathy. Humanizing the graft’s anticoagulant surfaces plus careful management of anticoagulants in the recipient are the countermeasures. Teams will track platelet counts, hemolysis markers, and graft microvasculature on biopsy.
- Zoonosis and porcine endogenous retrovirus. The fear is a pathogen jumping species or a retrovirus inserting into human cells and replicating. Source animals are raised in designated pathogen-free facilities with biosecurity and surveillance. eGenesis goes further by inactivating many retroviral sequences at the genome level. Trial protocols include long-term recipient monitoring, polymerase chain reaction assays for porcine viral nucleic acids, and family counseling about precautions while data accumulates. The risk is not zero, which is why surveillance is intensive.
- Endocrine cross-talk. A kidney is more than a filter. It helps regulate blood pressure via the renin-angiotensin system, produces erythropoietin to stimulate red blood cell production, and activates vitamin D. The question is whether pig proteins land correctly on human receptors at physiologic levels. Early cases suggest blood pressure and hemoglobin can be supported, but trial data will need to show consistent control without harmful off-target effects.
- Infection control while immunosuppressed. Even if no pig-specific pathogen crosses over, human pathogens exploit immunosuppression. Cytomegalovirus, BK virus, and opportunistic fungi are standard risks in human allotransplant. Expect aggressive prophylaxis, preemptive screening, and fast escalation protocols.
The tradeoffs should be measured against the real harms of staying on dialysis: vascular access infections, cardiac strain, amyloidosis, and a steep mortality curve. Safety is relative, not absolute, and the trial designs reflect that.
What the trials have to prove
Regulators and clinicians are aligned on practical endpoints. Watch for these signals over the next 6 to 24 months. This is consistent with the translational shift described in PER data’s 2025 human trials pivot.
- Graft survival at fixed time points, especially 3, 6, and 12 months. A clear separation from dialysis outcomes is the goal. That means living off dialysis with stable organ function.
- Kidney performance. Estimated glomerular filtration rate, creatinine trajectory, and urine output in the first week are bellwethers. Sustained function with minimal proteinuria will matter most.
- Rejection and biopsy pathology. Fewer biopsy-proven rejection episodes and mild histology under standard immunosuppression would indicate that the edits and thymus strategies are working.
- Infection and oncogenic risk. Incidence of serious infections, any detection of porcine viral genetic material, and early cancer signals under the immunosuppression regimen will be tracked for years, not months.
- Patient-centered outcomes. Time out of the hospital, ability to work or care for family, blood pressure control, and anemia correction are not soft endpoints. They are the daily reality of success.
Two companies will provide complementary evidence. United Therapeutics is beginning with a 10-gene edited pig kidney program that can scale if the first six patients do well. eGenesis has reported multiple expanded-access transplants since 2024 and, by September 2025, received federal clearance to launch a formal trial of its heavily edited, virus-inactivated source kidneys. The combination of approaches increases the chance that one or both will hit a workable safety-function balance.
How fast could this reach approval
Timelines in transplantation are measured in survival curves, not headlines. A realistic arc looks like this.
- 2025 to mid 2026. Pilot cohorts of 3 to 6 patients establish the surgical workflow, early safety, and dosing of immunosuppression. Expect protocol updates as teams learn from the first month of function and biopsy data.
- Late 2026 to 2027. Expansion to dozens of patients across more centers. This is where rare adverse events surface and where manufacturing reliability gets tested. If graft survival past six months is common and infection signals remain manageable, regulators can map a path to a limited label.
- 2027 to 2029. First approvals are plausible for narrowly defined populations: older dialysis-dependent adults who are unlikely to receive a human kidney in time or patients who are medically ineligible for allotransplant. Broader labels will wait for multi-year data.
The gating factor is not only biology. It is supply chain. Designated pathogen-free breeding facilities, surgical training, and 24-hour logistics must scale together. United Therapeutics has already opened clinical-scale facilities for source animals, and eGenesis is building out capacity. Hospitals will need dedicated xenotransplant pathways with infection prevention, specialized pathology, and data infrastructure to satisfy long-term monitoring requirements.
The cost, the capacity, the care model
No one should pretend this will start cheap. Early xenokidneys will carry research-level costs. Over time, economics can bend through predictable production, fewer canceled surgeries, and less time on dialysis. Payers will compare the total cost of care for an 18-month functioning xenograft to the cost of dialysis and hospitalizations over the same period. If a xenokidney holds for two to three years in a high-risk patient who would not have received a human kidney, the value case becomes compelling. Ultimately, scale is the best price control.
A practical care model looks like this:
- Pretransplant assessment focused on antibodies to human and pig antigens, infection history, and cardiovascular risk.
- Surgery at accredited centers with 24 to 48 hours of intense monitoring for early rejection and coagulation issues.
- A standardized immunosuppression protocol with rapid tapering if thymus co-transplantation achieves tolerance signals.
- Weekly surveillance in the first two months with polymerase chain reaction tests for porcine viral sequences, then tapering to monthly and quarterly visits.
- A data registry that captures function, infections, and quality of life for at least five years per recipient.
What to watch, and what to do now
- Clinicians. Identify dialysis-dependent patients who are either ineligible for human kidneys or unlikely to receive one for several years. Educate them about trial eligibility, surveillance obligations, and the specific risks of xenotransplant. Build relationships with centers running the studies.
- Hospital leaders. Stand up xenotransplant standard operating procedures for infection control, operating room workflow, and pathology. Invest in staff training. Patients will ask about this; be ready to answer with process and not just hope.
- Policymakers. Modernize surveillance frameworks so long-term viral monitoring is funded and interoperable. Clear guidance reduces fear and speeds evidence generation.
- Payers. Model the cost of 12, 18, and 24 months of dialysis avoided. Include hospitalization offsets and productivity. Create pilot coverage with evidence development so patients are not bankrupted by being pioneers.
- Patients and families. Ask your nephrologist about trials and expanded access criteria. Understand the commitment. The ask is not just a surgery. It is years of follow-up to help prove or disprove a new therapy that could help many others.
The bottom line for longevity
The modern longevity stack is full of molecules and metrics. Xenokidneys are different. They are hardware. They remove a major cause of death by replacing a failing part with a working one. In 2025, that proposition crossed from theory into the clinic with the first regulated studies and a string of compassionate-use cases that showed real function. The biology is no longer a black box. It is a set of targeted edits, smarter immunology, and industrial biosecurity applied to a very old medical problem.
Alongside blood-based interventions like plasma exchange’s 2025 pivot, xenokidneys represent a complementary, hardware-first path. If the next cohorts demonstrate steady function, tolerable infection rates, and durable survival, the first approvals will not be science fiction. They will be a scheduling challenge. A decade from now, a patient starting dialysis at age 65 might expect a manufactured organ rather than a multiyear wait. Among near-term longevity interventions outside metabolic therapies, that is as practical as it gets.
The story to watch is not whether pig kidneys can work once. That has been seen. The story is whether they can work reliably, safely, and at scale. Trials now underway will answer that question. If they do, life expectancy curves will bend in a way only organ replacement can deliver: by simply restoring what the body has lost, and letting the rest of biology do its quiet work.