Plasma Exchange’s 2025 Pivot: Human Data and Expansion
A randomized human study in 2025 reported multi-omics rejuvenation signals after therapeutic plasma exchange, strongest when IVIG was added. Here is what the data means, how clinics plan to scale, and what the next randomized trials must prove.
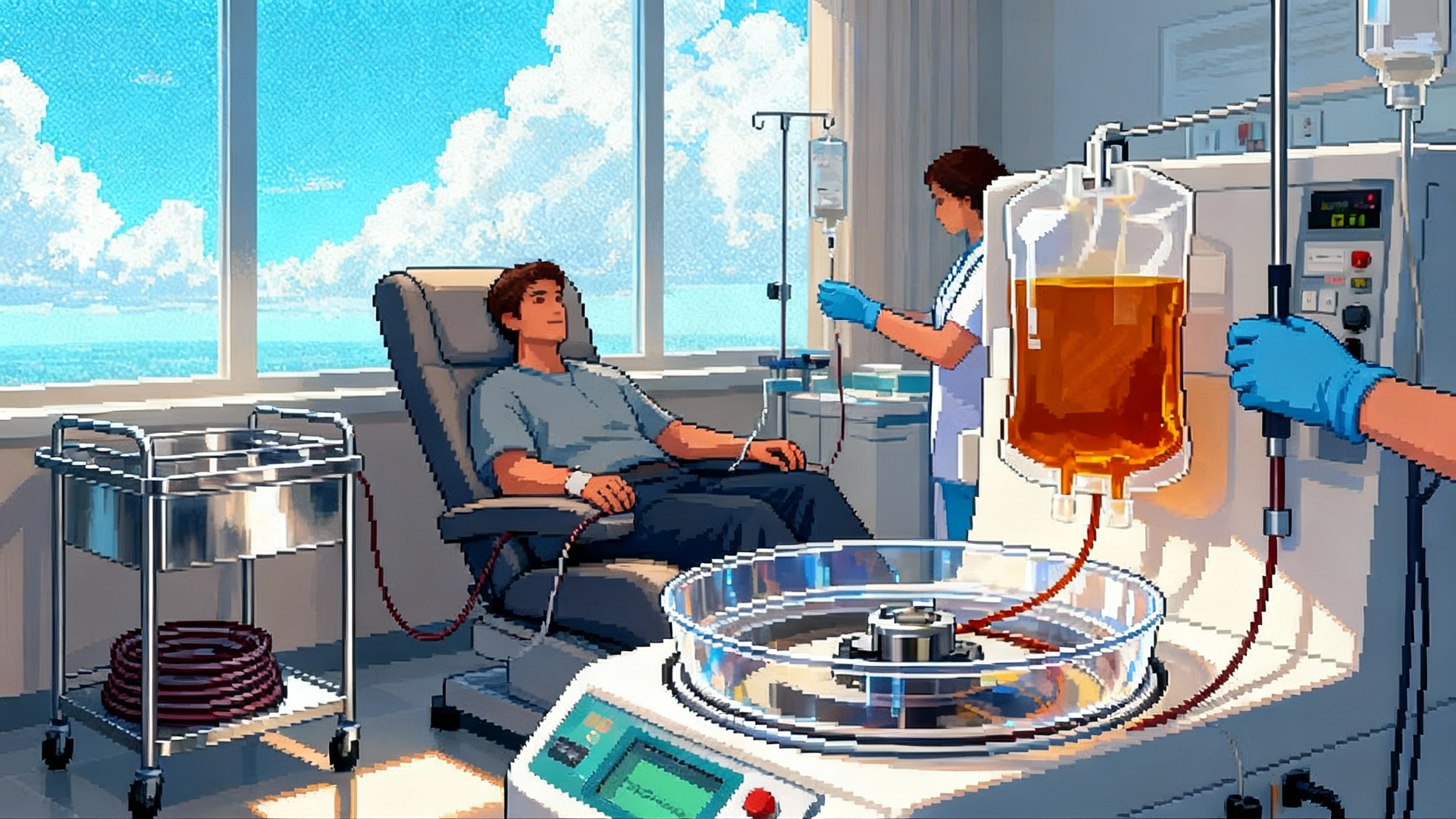
The inflection point everyone has been waiting for
For a decade, therapeutic plasma exchange sat in the shadow of the young blood narrative, a mélange of rodent parabiosis experiments and Silicon Valley myth-making. In 2025, the story changed. A single-blinded, randomized, placebo-controlled human study reported that repeated therapeutic plasma exchange in adults over 50 produced measurable shifts in biological age across multiple omics layers, with the strongest effects in the arm that added intravenous immunoglobulin. The authors profiled epigenetic clocks, proteomics, metabolomics, glycomics, cytokines, immune cell composition, and the inflammation-centric iAge score. Compared with placebo, participants on the most intensive regimen showed consistent rejuvenation signals across 15 epigenetic clocks, and adverse events were few. You can read the study summary on PubMed as a randomized multi-omics TPE trial.
That paper does not crown therapeutic plasma exchange as an anti-aging cure. It does something more useful. It supplies a coherent, human, multi-omics signal to replace hand-waving about diluted bad stuff. It also clarifies how to design the next wave of trials that must move from molecular clocks to function people can feel and regulators will recognize. Similar translational pivots defined 2025 elsewhere, including Klotho therapy’s 2025 pivot.
What therapeutic plasma exchange actually does
The procedure is straightforward in concept. Blood is withdrawn through an IV or catheter, plasma is separated and discarded, and the cellular components are returned to the circulation suspended in a replacement solution. In most protocols that replacement is albumin in saline. Each session typically exchanges close to one patient plasma volume. The immediate physiological effects fall into two categories:
- Dilution of circulating factors that sustain chronic, low-grade inflammation. That includes cytokines, chemokines, complement components, and protein aggregates bound to albumin. Removing a substantial fraction at once perturbs the feedback loops that lock the immune system into an age-associated pro-inflammatory set point.
- Restoration of proteostasis capacity. Fresh albumin is not merely a volume expander. It is a major carrier and buffer for fatty acids, hormones, and toxins. Replacing older, glycated, oxidized albumin with functional albumin improves binding and clearance dynamics. In plain terms, the body gets better at carrying and disposing of molecular clutter.
Together, these effects can reset aspects of immune signaling and reduce inflammaging. Downstream, that can alter transcriptional and epigenetic programs, change glycan patterns on antibodies and other proteins, and shift metabolic intermediates. A single intervention that touches multiple hallmarks of aging is not magic. It is system-level housekeeping.
What changed in the 2025 trial
The 2025 Aging Cell paper randomized 42 adults over 50 into several regimens: biweekly plasma exchange with IVIG, biweekly without IVIG, monthly exchange, and a placebo control. The trial was single-blinded, with randomization keyed to entry dates to keep logistics feasible. The team measured a large panel of multi-omics biomarkers repeatedly over time.
Key readouts, in simple terms:
- Broad epigenetic clock shifts. Fifteen DNA methylation clocks, including first- and second-generation clocks and rate-of-aging models, moved toward younger values in treatment arms compared with placebo. The composite effect size was greatest in the biweekly TPE plus IVIG cohort.
- Proteomic and cytokine recalibration. Proteins associated with chronic inflammation and immune dysregulation decreased toward youthful ranges, again more strongly with the combination regimen.
- Immune composition and iAge. Immune cell subset proportions shifted toward more resilient profiles, and the iAge measure dropped in parallel with proteomic changes.
- Heterogeneity with a pattern. Individuals with higher baseline risk markers, such as elevated glucose, tended to experience larger biomarker improvements. This suggests the intervention may have the biggest impact in metabolically or immunologically older participants.
- Safety and tolerability. Across long-term follow-up, the authors report only two discontinuations and one event linked to IVIG. The adverse event profile otherwise looked like standard apheresis practice.
A headline estimate from the study: the average biological age reduction in the TPE plus IVIG arm was about 2.6 years, while TPE alone averaged around 1.3 years. Those are statistical shifts in clocks and correlated omics, not claims of added calendar years. They are also relative to a placebo group that did not experience rejuvenation signals across the same measures.
Why the biology makes sense
The findings are consistent with a dilution-and-replacement model that has matured over the past several years. Aging blood contains higher concentrations of pro-inflammatory mediators, misfolded proteins, immune complexes, and albumin-bound toxins. Exchange removes a large portion of that load in one go. The immune system, relieved of the inflammatory bath, can recalibrate. The liver, receiving a wave of fresh albumin, can restore binding and shuttling functions that improve metabolic homeostasis. Glycomic remodeling of antibodies tracks with this shift because antibody glycans are dynamic and reflect inflammatory tone. These upstream changes ripple into epigenetic marks and transcriptional programs.
A simple mental model helps. Imagine your plasma as the medium that bathes every tissue and instructs immune cells. If you remove much of the distorted instruction set and replace it with a cleaner signal, many downstream processes can reset together. That is the systems reason multi-omics shifts appear coordinated rather than scattered.
Safety profile and practical trade-offs
Plasma exchange is not new. It is widely used for autoimmune and hematologic indications and delivered by trained apheresis teams. Still, a longevity use case raises different risk-benefit math.
- Procedure risks. Vascular access, anticoagulation with citrate, and fluid shifts can cause hypotension, lightheadedness, and transient hypocalcemia. These are generally manageable with monitoring and calcium supplementation. Rare complications include allergic reactions to albumin or IVIG, line infections, and bleeding in patients with coagulation issues.
- Immune effects. Repeated exchanges transiently lower immunoglobulins and complement proteins. Adding IVIG replenishes antibodies but introduces its own rare risks, such as aseptic meningitis or thrombosis in predisposed individuals. People with chronic infections, severe anemia, unstable cardiovascular disease, or uncontrolled bleeding disorders are typically poor candidates.
- Cost and access. Outside of approved indications, plasma exchange for longevity is cash-pay in the United States. Sessions require specialized equipment, nurses trained in apheresis, and medical oversight. Out-of-pocket costs are material and cumulative if done frequently. Insurance coverage is unlikely without disease-specific indications.
In short, the adverse event profile appears acceptable in experienced hands, but this is not a spa treatment. Patient selection, dose scheduling, and post-procedure monitoring matter.
The venture-backed clinic push
Human data invites capital, and capital builds access. Within weeks of the Aging Cell paper, Circulate Health announced a seed round led by Khosla Ventures and outlined plans to expand a partner network of clinics across multiple states. The company cites the trial’s multi-omics outcomes and positions TPE as a platform to address chronic inflammation and age-related disease. See details in the Khosla-led seed funding round.
The emergence of cash-pay TPE for longevity will feel familiar. Concierge labs, body scans, and peptide programs have already defined the category. The difference here is that TPE requires regulated devices, trained staff, and medical-grade supply chains for albumin and IVIG. Those barriers raise the floor on quality and the ceiling on cost. They also create incentives to standardize protocols and run trials that justify the price and pave a path to reimbursement for disease-focused use cases.
What to test next: designing RCTs that matter
The 2025 study built a convincing omics narrative. The next trials must tell a clinical story.
- Population and stratification. Enroll adults 55 to 80 with measurable inflammatory burden or metabolic dysregulation, not the already-optimized. Baseline stratification by biological age rate, glycemic status, and inflammatory markers can enrich for responders and test the heterogeneity seen in 2025.
- Arms and dose. Compare at least three regimens: biweekly TPE plus IVIG, biweekly TPE without IVIG, and a plausible sham or low-dose control. Include a maintenance phase to answer the durability question and a crossover design to minimize dropout and ethical concerns if benefits emerge.
- Primary endpoints that mean something. Choose functional outcomes that regulators already respect and that people notice in daily life: gait speed, the Short Physical Performance Battery, 6-minute walk, VO2peak in a subcohort, handgrip strength, and a composite frailty index. For cognition, use a validated composite sensitive to early change, such as a PACC-style battery in a metabolically impaired subgroup. Pre-specify minimal clinically important differences. Lessons on powering and adherence from the PEARL rapamycin trial can help teams avoid common pitfalls.
- Secondary endpoints that explain why. Retain the multi-omics battery, but treat it as mediation analysis tied to the primary functional outcomes. Add albumin quality metrics, complement function, and antibody glycan signatures as mechanistic bridges.
- Safety and adherence. Track immunoglobulin levels, infection incidence, bleeding events, blood pressure instability, and adverse reactions to albumin or IVIG. Monitor iron indices and hematocrit to ensure no drift toward anemia with frequent sessions.
- Follow-up and durability. Assess outcomes at 3, 6, and 12 months post-intervention to learn whether the system reset persists, decays, or requires maintenance.
- Sample size and power. The 2025 trial suggests effect sizes in biological clocks that are large, but functional gains will be smaller. Power for 0.1 to 0.15 m/s improvements in gait speed or 30 to 50 meter changes in 6-minute walk in higher-risk participants. Expect 15 to 20 percent attrition and plan accordingly.
If a trial like this shows clinically meaningful improvements with acceptable safety, the conversation changes from curiosity to care pathway.
From biomarkers to benefits regulators recognize
Epigenetic clocks, proteomic signatures, and composite omics scores are useful discovery tools and potential surrogates. Today, they are not accepted endpoints for approvals or coverage decisions in the United States. A pragmatic regulatory path for TPE in longevity-adjacent care looks like this:
-
Replication with pre-specified analyses. Run a second single-blind or double-blind multicenter study that confirms the 2025 omics findings and pre-specifies which clock or multi-omics composite will be used for hypothesis testing. Transparency about analysis plans is key to avoiding p-hacking concerns.
-
Indication scoping. Rather than seeking a vague aging claim, target age-associated conditions where inflammaging is a major driver and where TPE is biologically plausible. Examples include frailty in type 2 diabetes, sarcopenic obesity with systemic inflammation, or mild cognitive impairment with elevated inflammatory markers. Regulators already have mental models for these conditions, and parallels exist with the St. Jude frailty trial.
-
Pivotal trials with functional primaries. For the chosen indication, power a randomized trial on accepted functional endpoints and quality-of-life measures. Use the omics panel as a secondary to support a mechanism-of-action story. If a composite endpoint is needed, pre-negotiate it with the agency in an end-of-phase meeting or a Q-submission.
-
Safety and risk management plan. Build a post-market active surveillance registry across all partner clinics to capture adverse events, durability of benefit, and real-world adherence. A uniform data model and independent safety board will build trust.
-
Reimbursement strategy. For Medicare-aged populations, pursue coverage with evidence development tied to the pivotal. Define coding pathways that reflect the use of existing apheresis devices and albumin products, but also the additional monitoring and multi-omics testing. Related policy shifts around lab-developed tests are summarized in the FDA LDT rollback on biomarkers.
-
Surrogate maturation. In parallel, submit biomarker qualification packages for omics composites most predictive of functional gains, so that over time the field can shorten trials without sacrificing credibility.
None of this requires inventing a new device class. It requires translating a procedure with known operating characteristics into indications where outcome measures are both meaningful and measurable.
Who should consider TPE now, and who should not
Until functional data arrive, TPE for longevity is an elective bet best suited to clinical trial participation or carefully selected patients under medical supervision.
- Reasonable candidates today might include older adults with clear inflammatory burden, metabolic syndrome, or immunosenescence markers who are already optimized on lifestyle and standard of care and who understand the evidence gaps.
- Poor candidates include those with significant anemia, bleeding or clotting disorders, uncontrolled infections, unstable cardiovascular disease, or those unable to tolerate volume shifts or anticoagulation.
- Patients on study protocols should have labs tracked before and after sessions, including calcium, electrolytes, immunoglobulins, fibrinogen, iron indices, and inflammatory markers, and should receive calcium prophylaxis when appropriate.
The 2026 proof everyone will trust
The field is one well-designed randomized trial away from a decisive answer. If biweekly TPE with or without IVIG can move a frailty score or gait speed in higher-risk older adults, with safety akin to standard apheresis, TPE graduates from intriguing biomarker modulator to a healthspan therapy with a clinical credential. If it cannot, the 2025 omics shift will be remembered as an elegant systems biology experiment that clarified how blood-borne signals shape aging, but not a basis for routine care.
Either way, 2025 marks the moment therapeutic plasma exchange stops being a story about young blood and starts being a story about human data, clinical design, and operational excellence. That is a better story for patients, for clinicians, and for the science of extending healthy years.