Cross-organ PER data mark 2025 pivot to first human trials
Cross-organ signals from Life Biosciences at ARDD 2025 move partial epigenetic reprogramming from hype to clinical planning. Here is what it means for delivery, safety, endpoints, first indications, and regulatory strategy.
Why ARDD 2025 felt like a turning point
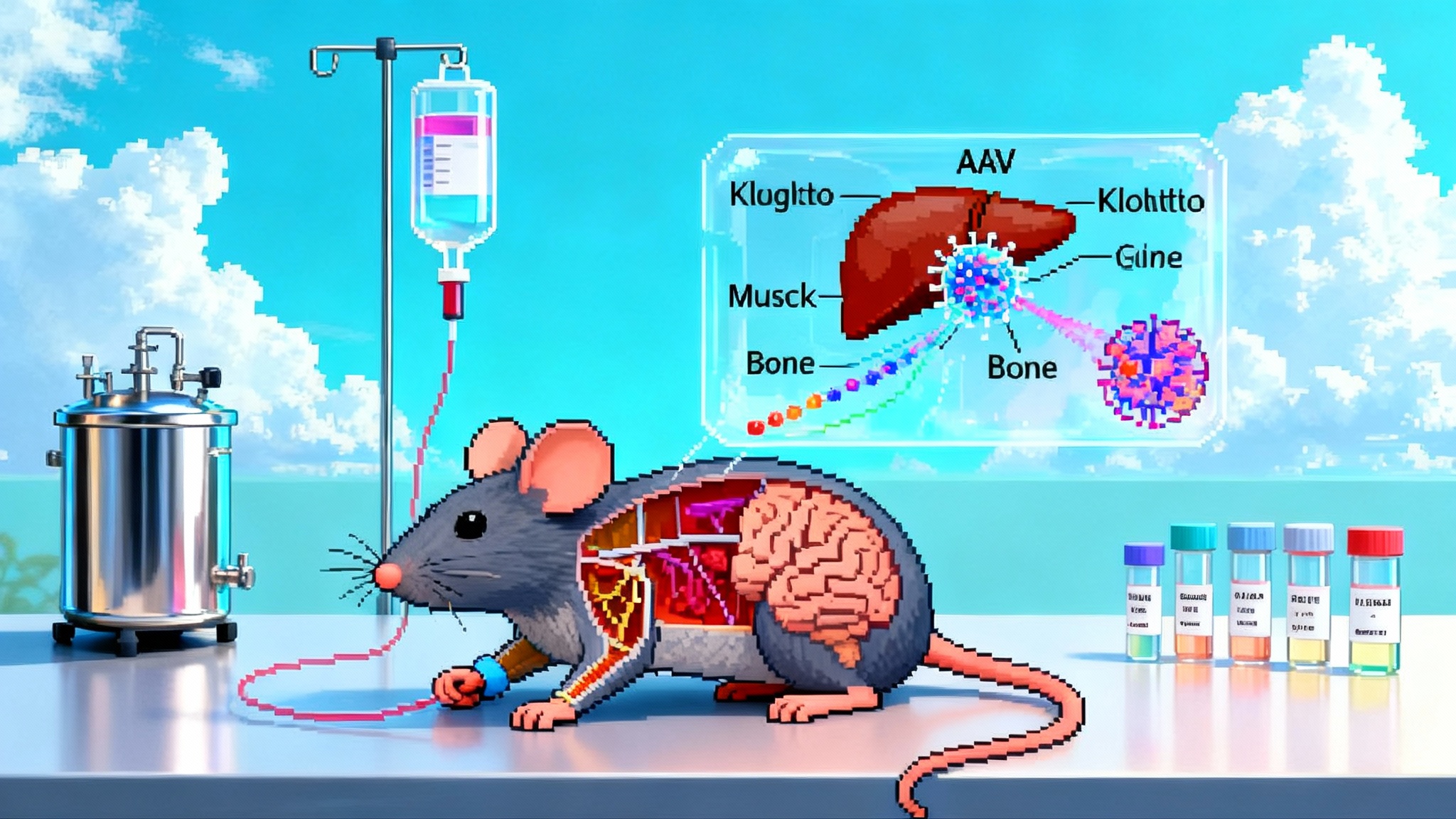
At the 12th Aging Research and Drug Discovery meeting in Copenhagen in late August 2025, Life Biosciences shared preclinical readouts that stitched together an eye and a liver story into one central theme. Their partial epigenetic reprogramming platform delivered OSK transcription factors and produced signals in a nonhuman primate model of optic neuropathy and in a mouse model of metabolic dysfunction-associated steatohepatitis. The company’s summary highlighted restoration of methylation patterns linked to neuronal regeneration in the eye and improved liver biomarkers across a standard MASH model, with ER-100 for optic neuropathies now targeted for first-in-human in early 2026. Those claims are detailed in Life Biosciences’ ARDD release, which is the best primary bridge between the conference stage and public record (ARDD 2025 data and timelines).
For longevity medicine, the significance is not that one company showed positive graphs. It is that cross-organ signals push partial reprogramming from a lab curiosity into a program strategy with concrete clinical paths. One set of eyes and one liver do not make a field, but they set a trajectory and a countdown clock.
What the new results actually say
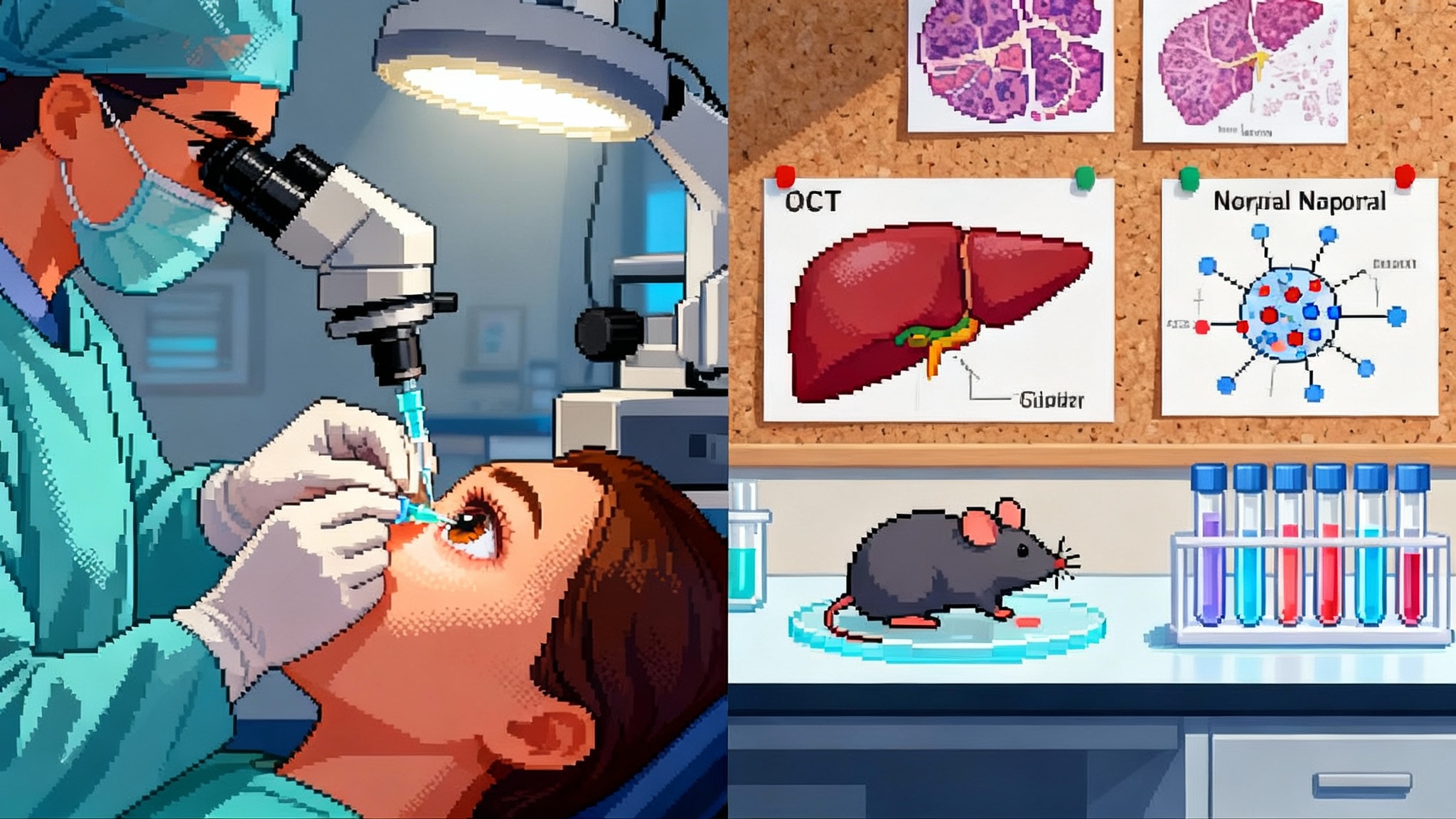
- Optic neuropathy model: In nonhuman primates with NAION-like injury, ER-100 was reported to restore DNA methylation patterns with enrichment in neuronal regeneration pathways. These epigenetic shifts were paired with prior functional readouts in the same model, including improvements in pattern electroretinography and axon survival.
- Liver model: In a well-characterized MASH mouse model, ER-300 improved a panel of biomarkers, including transaminases, bile acids, liver weight, histologic steatosis, and lipid droplet burden. A single biomarker can wobble for many reasons; a multi-parameter liver profile rising and falling in the right directions is more persuasive.
- Timelines: The company now guides to first-in-human studies for ER-100 in the first quarter of 2026 across two optic neuropathy indications. That is a concrete mark on the calendar for clinical aging biology.
Taken together, these data do not prove rejuvenation in a clinical sense. They do suggest that OSK-based partial reprogramming can induce measurable molecular and functional changes in at least two tissues that fail with age. That is the definition of a 2025 inflection point.
Delivery choices now matter more than slogans
Partial epigenetic reprogramming is not an ingredient; it is a delivery-dependent program. The same transcription factors can look precise or risky depending on how and where they are deployed. Two viable routes stand out.
AAV for the eye
- Vector and route: An AAV2 vector with OSK delivered intravitreally aligns with the way retina gene therapy has been de-risked. The eye is a small, compartmentalized space, immune privileged relative to systemic circulation, and already home to an approved AAV product class. Local dosing limits systemic exposure and allows tight monitoring through imaging and electrophysiology.
- Control of expression: Inducible systems anchored to doxycycline or similar switches give investigators a temporal dial. You can pulse OSK expression around injury windows or taper after signal appears. This is not a theoretical advantage; it is a control that lets teams minimize dedifferentiation risk while maximizing plasticity windows.
- Redosing: Intravitreal AAV can trigger neutralizing antibodies that complicate redosing. That makes the front-loaded dose planning and on-off control more important. It also raises the value of contralateral eye designs when ethical and feasible.
Nonviral options for the liver
- Why nonviral is attractive: The liver naturally takes up lipid nanoparticles, which makes mRNA or RNP cargo delivery efficient. Nonviral delivery avoids vector genome persistence and enables repeat dosing. For partial reprogramming, where tight time windows may be safer, transient expression is a feature rather than a bug.
- Practical constraints: OSK is three open-reading frames. Packaging them as separate mRNAs increases complexity, while polycistronic strategies can bloat cargo size. Manufacturing and release testing need to be dialed for consistency, and translation kinetics differ from AAV-mediated gene expression.
- Redosing and safety: Repeatable nonviral dosing allows iterative titration of effect. That unlocks dose exploration without long tail expression, which is important if tumorigenicity risk is tied to exposure duration rather than momentary peak.
The hybrid horizon
Expect a hybrid future where AAV dominates localized tissues like the eye and nonviral vectors compete or dominate systemic targets like liver and muscle. A platform company will likely need both tools and a decision framework that looks beyond transduction efficiency to clinical workflow realities like procedure time, monitoring cadence, and rescue options. For a parallel on delivery strategy and product framing, see the Lilly’s Verve LDL editing deal.
Tumorigenicity safeguards and the art of the dosing window
Every discussion of reprogramming starts with the same safety question. Tumors are not an abstract risk. What matters is engineered restraint.
- Factor selection: Excluding c-Myc reduces oncogenic risk while retaining chromatin opening and identity-preserving activity. OSK rather than OSKM is now table stakes for clinical reprogramming.
- Spatial restriction: Tissue-specific promoters and microRNA target sites can bias expression into the intended cell types and silence it elsewhere. In the retina, RGC-targeted strategies reduce off-target expression in photoreceptors or glia. In the liver, hepatocyte-favoring promoter elements and miRNA response elements can limit non-parenchymal cell exposure.
- Temporal control: Inducible systems provide on-off switches measured in hours and days rather than the weeks to months of AAV decay. Pulsed regimens can try to harvest plasticity without erasing identity. Think of reprogramming like heat. Enough warms and loosens the system; too much melts it.
- Safety brakes: Incorporating a kill switch such as inducible caspase 9 provides a last line of defense. It costs vector real estate and manufacturing complexity, but it can be the difference between a manageable event and a program-ending pause.
- Window finding: In optic neuropathies, there may be early prevention windows after ischemic injury and later rescue windows once damage is established. Preclinical data showing benefit in both time frames are valuable because clinic will enroll people across that spectrum.
The preclinical path should treat safety as a multi-layered problem. If a program relies on only one safeguard, it has not been engineered enough.
How to prove “rejuvenation” in people
No regulator will approve a label that says a drug makes you younger. What you can prove is improvement in function and a shift in validated disease biomarkers, while showing concordant changes in aging-linked molecular readouts. The practical formula looks like this.
- Lead with function: In the eye, functional endpoints such as best corrected visual acuity, contrast sensitivity, visual fields, pattern ERG, and ganglion cell complex thickness by OCT will anchor decisions. In the liver, regulators still look at histology and fibrosis progression along with well-defined serum panels. Functional claims must tie to how patients see, feel, or function.
- Add disease-specific structure: In ophthalmology, durable changes in RNFL thickness or macular parameters on OCT support the story. In hepatology, noninvasive imaging such as MRE, elastography, and multiparametric MRI, plus transaminases and bile acids, create a corroborating scaffold.
- Use epigenetics as supportive evidence: DNA methylation age estimates and locus-level methylation at injury or identity genes can be prespecified as exploratory or secondary endpoints. They should be run in centralized labs with locked analysis plans and prespecified statistical thresholds. The aim is to show that functional gains align with epigenetic directionality, not to claim approval-level surrogacy.
- Pre-register the hierarchy: Put functional endpoints in the primary hierarchy, place disease biomarkers next, and keep epigenetic clocks in a pre-declared exploratory tier. If the program wants clocks to matter in the label later, plan multi-trial replication and a consortium path toward qualification. For the regulatory backdrop on lab tests and biomarkers, see the FDA LDT rollback on biomarkers.
- Embrace composites carefully: Composite endpoints can amplify signal but can also hide heterogeneity. If you use a composite, commit to reporting each component separately and justify the clinical weighting.
The key is coherence. Regulators and clinicians will respond if functional improvement, structural change, and epigenetic directionality all point the same way with prespecified analysis.
Likely first indications and why they make sense
- Non-arteritic anterior ischemic optic neuropathy: NAION offers an acute injury window and a clear functional readout. It also presents a patient population hungry for options and a feasible intravitreal procedure path. The contralateral eye can serve as a within-subject comparator in some designs.
- Glaucoma: Chronic damage makes endpoints trickier and slower, but a large population and well-tooled imaging and functional measures make it attractive. Risk tolerance is lower given healthy fellow eyes and chronic disease context.
- MASH with early fibrosis: The liver data focus on biomarker improvement in an established model. Clinically, stage 1 to 2 fibrosis with active steatohepatitis may offer a window where transient OSK expression could reset lipid handling and inflammation without chasing cirrhosis. The field will expect careful alignment with histologic or validated noninvasive endpoints.
Ocular indications are the natural beachhead because of local delivery, established imaging, and existing gene therapy precedents. The liver is a logical runner-up if nonviral delivery can achieve reproducible, titratable expression.
How regulators may view organ-specific claims tied to aging biology
Regulators do not approve mechanisms. They approve benefits in specific conditions. The safest posture is to frame aging biology as the rationale for a disease trial, not as the object of the claim. The FDA’s guidance on human gene therapy for retinal disorders lays out expectations for product characterization, preclinical models, and the functional endpoints that matter in the clinic (FDA retinal gene therapy guidance).
Practical implications for partial reprogramming programs:
- Define the patient, not the philosophy: Target NAION, glaucoma, or MASH with precise inclusion criteria and baseline risk. Do not try to enroll aging.
- Specify control of expression: Detail inducible elements, dosing schedules, and planned pauses. Regulators want to understand how you avoid over-reprogramming.
- Commit to long-term follow-up: Gene therapy class effects require multi-year surveillance. Even with nonviral vectors, reprogramming mechanisms will draw scrutiny for delayed adverse events.
- Use epigenetics as supportive evidence: DNA methylation and transcriptional signatures can enrich the biological narrative but should not carry the primary proof burden until qualified. If your team aims to elevate an epigenetic measure toward a surrogate, begin the biomarker submission pathway early and plan replication across studies.
- Consider expedited programs thoughtfully: RMAT or Breakthrough designation can be realistic for vision recovery in a disease with no approved therapies if early human data show magnitude and durability. Mechanism will not unlock an expedited path on its own; patient-centered effect will.
In short, expect regulators to welcome a mechanistic discussion of aging but to anchor approvals in classic disease endpoints. If you want an aging-linked biomarker to matter, do the hard, multi-trial work to qualify it.
What this means for the next 12 months
- Clinical trial design locks: For ocular programs, finalize powering assumptions around BCVA and field measures, define rescue windows, and codify inducible dosing plans. For liver work, decide whether to pursue histology-based endpoints or a composite noninvasive package with regulatory alignment.
- Manufacturing choices: AAV2 for the eye is the pragmatic choice today. For liver, decide whether nonviral delivery can support consistent multi-dose regimens and whether cargo architecture for OSK can be made robust at scale.
- Safety playbook: Build tiered safeguards into the vector, protocol, and monitoring plan. Predefine stopping rules based on on-target but excessive reprogramming signals, not only classic adverse events.
- Biomarker governance: Pre-specify epigenetic assays, lock analysis plans, and budget for central lab replication. If the goal is eventual qualification, begin documentation in the language regulators use for biomarker submissions and coordinate with movements like the plasma exchange 2025 pivot that highlight the value of clean human readouts.
- External validation: Encourage independent labs to replicate key animal findings with blinded analyses and shared reference standards. The credibility return is worth the coordination cost.
The bottom line
Partial epigenetic reprogramming is moving from promise to plans. The ARDD 2025 data give the field a narrative that connects the eye and the liver through a single mechanism and a common set of engineering questions. AAV dominates where anatomy favors local control; nonviral options rise where repeatability and titration are decisive. Safety is a design problem, not an afterthought, and dosing windows are as important as dose. Proof of human rejuvenation will come, if it comes, by pairing functional recovery with coherent molecular directionality in diseases that matter to patients. Regulators are ready to evaluate that case when it is framed organ by organ, endpoint by endpoint.
If the next year brings clean ocular dosing, early functional signal, and a reproducible epigenetic shift that tracks with vision, 2026 will be the year partial reprogramming finally meets the clinic on clinical terms.