Lilly’s Verve buy puts one-shot LDL editing on the longevity map
Lilly’s July 2025 purchase of Verve puts one-shot PCSK9 base editing on a fast track from lab signal to real-world prevention. Here is what the data showed, the risks, the regulatory path, and what to watch next.
The moment LDL gene editing went mainstream
On July 25, 2025, Eli Lilly closed its acquisition of Verve Therapeutics, folding one of the most closely watched gene editing programs into a pharma giant’s pipeline. In a few sentences, Lilly framed the ambition clearly: deliver lifelong cardiovascular risk reduction with a one-and-done treatment. The deal makes PCSK9 base editing a front line story for prevention, not a speculative lab project, and it accelerates the timeline for a therapy designed to flip a single DNA letter so the liver stops making a protein that pushes LDL cholesterol up. The acquisition is now official and public, which moves the conversation from if to when and how. Lilly completes acquisition of Verve Therapeutics.
From daily adherence to durable risk reduction
Cardiovascular prevention has always wrestled with time. Risk accumulates day by day as LDL particles cross arterial walls, and benefit accumulates only if patients keep taking treatment. Statins work, yet half of patients stop within a year. PCSK9 antibodies are powerful but require ongoing injections. siRNA like inclisiran extends the dosing interval, yet adherence still matters. A single-dose in vivo editor promises to change the math by replacing adherence with durability. If a one-time therapy can cut LDL in half and keep it there, the long arc of atherosclerosis could bend much earlier and for longer.
This is not just a convenience story. It is a population risk story. The lifetime exposure to LDL, sometimes simplified as the area under the LDL curve, predicts events. Lowering LDL earlier and maintaining that reduction produces bigger absolute benefits than starting late. A durable therapy that locks in a lower baseline could help people keep cardiovascular risk at bay without a daily decision. For context on adherence tradeoffs, see our look at rapamycin trial insights and the uptake dynamics in GLP-1 muscle-sparing therapies.
What the Heart-2 data actually showed
The April 2025 Heart-2 readout from Verve’s VERVE-102 was the moment the field got hard clinical signals. In 14 participants across three dose levels, a single infusion produced dose-dependent drops in blood PCSK9 and LDL cholesterol. The mean LDL reduction was about 53 percent, with a maximum reduction of 69 percent in the 0.6 mg/kg cohort, at least through the first month of follow up. The company also reported no treatment-related serious adverse events and no clinically significant changes in liver enzymes or platelets across the assessed doses. That is the backbone of the story Lilly is now advancing, because it couples meaningful LDL lowering with early safety signals in humans. Heart-2 initial VERVE-102 data.
Several caveats matter. Fourteen people is a small sample, and these were early data with time-averaged changes from day 28 to the last available follow up. Durability beyond a few months, the shape of the dose response at 0.7 mg/kg, and performance in broader populations still need to be shown. Yet for a therapy that works by editing liver cells in vivo, seeing consistent, dose-related pharmacodynamics with early safety is exactly what you would hope to see at this stage.
How it works: PCSK9, base editing, and GalNAc-LNP delivery
-
The target. PCSK9 is a liver-produced protein that binds LDL receptors and promotes their degradation. Fewer receptors on hepatocytes means less LDL clearance and higher LDL levels. People born with natural loss-of-function mutations in PCSK9 run low LDL for life and have fewer coronary events. Turning PCSK9 off pharmacologically is already validated by antibodies and siRNA. Editing the PCSK9 gene seeks to make that suppression permanent in the liver.
-
The tool. VERVE-102 uses an adenine base editor, rather than a CRISPR nuclease that cuts both DNA strands. Base editing swaps one DNA letter for another without creating a double-strand break. For PCSK9, the guide RNA steers the editor to a specific DNA sequence, and the editor converts an A to a G in a spot that disrupts the PCSK9 gene. The result is a stop in PCSK9 production in edited hepatocytes. Because hepatocytes turn over slowly, and because the DNA change is copied when cells divide, the effect is designed to persist.
-
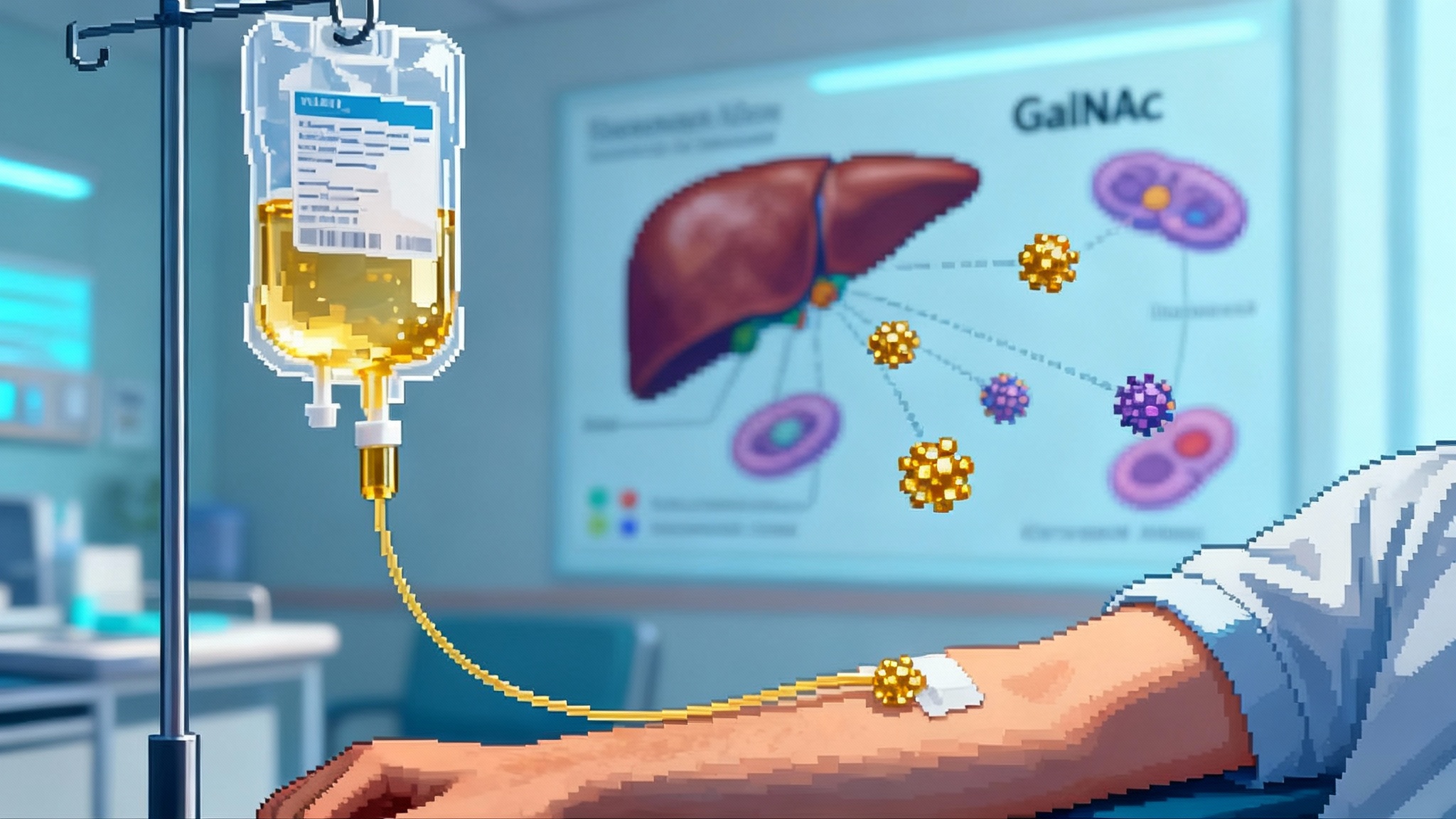
The delivery system. The editor is delivered as mRNA together with the guide RNA inside a lipid nanoparticle that carries a GalNAc ligand. GalNAc binds the asialoglycoprotein receptor on hepatocytes, which enhances uptake into the right cells. VERVE-102 differs from earlier constructs by using an updated ionizable lipid and the GalNAc targeting element. The aim is to boost potency in the liver and improve tolerability compared with first generation LNPs.
Put simply, the therapy is an infusion of two RNAs wrapped in a targeted nanoparticle. The liver takes up the package, translates the editor protein, and the editor makes a single-letter correction in PCSK9 to switch the gene off. After that, the liver returns to business as usual, only now with more LDL receptors on the surface and lower LDL in the blood.
Safety: where the risks live and how to manage them
The promise of a one-time edit comes with different risks than chronic drugs. Early data from VERVE-102 are encouraging, but regulators and clinicians will watch several categories closely.
-
Editing specificity. Off-target edits can occur if the guide RNA partially matches another site. Base editors also have a window where nearby bases could be changed, known as bystander edits. Companies address these risks with high-fidelity editors, stringent guide design, and extensive off-target testing in preclinical models. In humans, sensitive sequencing of targeted sites and unbiased screens can help quantify risk, but long-term surveillance will still be important.
-
Liver safety. LNP-based medicines can trigger transient transaminase elevations or platelet changes. The earliest VERVE-101 experience raised concerns that contributed to a pivot toward the VERVE-102 construct and targeting. The Heart-2 data to date did not show clinically significant changes in ALT or platelets in the first three cohorts. The 0.7 mg/kg cohort and longer follow up will be watched for any signal.
-
Infusion and complement activation. Rapid LNP infusion can sometimes activate complement pathways and cause infusion reactions. Heart-2 reported one grade 2 infusion reaction that resolved with acetaminophen. Infusion protocols, premedication strategies, and careful dose escalation can reduce risk.
-
Genomic permanence. A benefit of editing is durability. A consequence is irreversibility at the cellular level. If a rare safety issue appears late, you cannot turn the edit off. That raises the bar for early characterization of off-target profiles and supports long-term patient registries.
-
Immunogenicity. The base editor protein is foreign, and repeated exposure could be limited by immune responses. The design goal is to edit once and be done. If a second dose is ever needed, immune memory could complicate retreatment, although different delivery strategies or transient immunosuppression might help.
In this context, the safety trade is transparent. Millions of people tolerate statins and PCSK9 antibodies well, with decades of accumulated experience. A one-time editor has to meet a high standard, yet it does not need to be risk free to be worth it. If the effect is both deep and durable, a carefully selected population could accept a small, well-characterized procedure risk for lifelong benefit.
The regulatory path: LDL-C as a surrogate, plus gene editing obligations
There is helpful precedent here. The FDA accepts LDL cholesterol as a validated surrogate endpoint for hypercholesterolemia, which has supported traditional approvals for multiple drug classes. That means a program can show robust, durable LDL-C lowering in the intended population, with an acceptable safety profile, and secure approval without waiting for long cardiovascular outcomes trials to read out. Outcomes studies still matter, but they need not delay initial access in all settings.
At the same time, gene editing brings additional expectations. Programs that permanently change DNA in vivo typically include plans for long-term follow up to monitor for delayed adverse events, even when the delivery platform is non-viral. Manufacturing consistency and release testing for editor activity and off-target potential are more complex than for standard small molecules or antibodies. Regulators will also expect convincing evidence that the edit hits the intended fraction of hepatocytes at the commercial dose, since editing efficiency determines LDL lowering and durability. For adjacent policy context, see the testing and infrastructure implications in FDA LDT rollback and biomarkers.
If VERVE-102 continues to show stable LDL reductions in the 40 to 60 percent range, with low variability and a clean safety profile, a Phase 2 that uses LDL-C as the primary endpoint could be enough to support an approval path in high-risk populations, such as heterozygous familial hypercholesterolemia or very high-risk secondary prevention. A broader primary prevention label would reasonably require more data on safety, durability, and perhaps outcomes.
What success would imply for longevity
Longevity is not a single lever. It is the compound effect of avoiding the diseases that cut life short or healthspan short. Atherosclerosis is still the leading cause of death worldwide, and LDL exposure fuels it. If a safe, one-time edit can cut LDL in half for decades, the implications for healthy lifespan are large because benefit accrues slowly and cumulatively. The most important feature is not the peak LDL drop in month one, it is the level you live at for years without lapses.
This also reframes prevention strategy. Today, clinicians triage therapy intensity using calculated risk and the realities of adherence. A reliable one-shot option could prioritize lifetime risk, family history, and genetic markers. In families with heterozygous familial hypercholesterolemia, editing in early adulthood might prevent decades of arterial injury. In secondary prevention, a one-time edit could simplify complex regimens and remove a major driver of residual risk.
None of this eliminates the role of lifestyle, blood pressure control, or diabetes management. But it could shift LDL from a chronic management problem to a durable fix, which is the kind of change that moves population curves.
How it stacks up against statins, antibodies, and siRNA
-
Statins. Oral, cheap, and evidence rich, statins remain the backbone of prevention. They lower LDL about 20 to 60 percent depending on potency and patient response, but adherence problems are profound. Even small lapses shrink long-term benefit. Statins will remain first line because of cost and access. A one-time editor will need to find its niche where durability matters most and where patients can accept a procedural risk.
-
PCSK9 antibodies. Drugs like evolocumab and alirocumab lower LDL 50 to 60 percent and reduce events, but they require injections every two to four weeks or monthly. They are effective add-ons but remain underused due to cost and logistics. A one-shot alternative that delivers similar LDL reductions without ongoing dosing would be an attractive option for people who never start or never stick with antibodies.
-
siRNA. Inclisiran lowers LDL about 50 percent with dosing every six months after a loading phase. It narrows the adherence gap but does not erase it. Some systems will prefer this middle ground, especially if cost is favorable and infrastructure for gene editing is limited. Editing would offer deeper durability at the cost of a one-time procedure and long-term follow up.
If VERVE-102 delivers on consistency and safety, the comparison most people will make is to antibodies and siRNA on LDL reduction, then to statins on scale and cost. The unique pitch for editing is durability without adherence. The practical question will be which patients benefit enough, early enough, to justify the procedure and monitoring, and whether health systems choose to invest in early prevention to avoid later events.
Milestones to watch in the second half of 2025
The near term is busy. Verve and Lilly have said to expect the final dose escalation readout from Heart-2 in the second half of 2025, including the 0.7 mg/kg cohort and more durability data. The plan is to start a Phase 2 trial of VERVE-102 in the same window, now with the resources of a large sponsor and the possibility of United States enrollment. Watch for three things:
- Durability at six to twelve months for the 0.6 and 0.7 mg/kg cohorts, not just the first month. If LDL remains stably reduced, confidence rises that the edit is present in enough hepatocytes.
- Safety across more participants and higher doses, with particular attention to liver labs, platelets, and infusion reactions. A noiseless safety profile will carry outsized weight because the effect is permanent at the cellular level.
- Variability and proportion of responders. Even with a strong mean effect, payers and regulators will care whether a predictable fraction of patients reach a clinically meaningful LDL reduction at a single dose.
If these boxes get checked, a 2026 to 2027 pivotal program focused on LDL-C as the primary endpoint for high-risk groups looks plausible. Outcomes studies can run in parallel to support future label expansions.
What it would take to scale
The technology is powerful, yet the practicalities matter. Health systems will need infusion capacity, sequencing and lab workflows to confirm editing and monitor safety, and long-term registries for follow up. Pricing will be a central debate. A one-time therapy that replaces decades of medicines and clinic visits can justify a premium, but it must be aligned with payer budgets and patient access. Risk sharing models, durability warranties, or outcomes-based contracts could all show up here.
Manufacturing is another key piece. LNP and GalNAc conjugation have become more mature, but editor mRNA and gRNA require precise quality control. The fastest way to scale may be through a small number of centralized facilities with cold-chain distribution, then a hub-and-spoke network of certified infusion centers. If the experience is streamlined and the post-infusion monitoring is straightforward, patient adoption will come easier.
The bottom line
With Lilly now carrying Verve’s program, one-shot PCSK9 base editing has moved from emerging science to mainstream development. The Heart-2 signal was the catalyst, showing clinically meaningful LDL reductions after a single infusion with early safety that supported escalation. The next six months will test durability and consistency at higher doses and will shape a Phase 2 designed around LDL-C as a surrogate endpoint. If the data hold, the field could soon have a new tool that turns the hardest part of prevention, adherence over decades, into a single clinical moment. That is why this acquisition matters for longevity. It is not just a new medicine. It is a new way to control a major risk factor for a lifetime.