Klotho Goes Clinical: From Mouse Longevity to Human Trials
Two 2025 milestones moved the classic longevity gene into the clinic: peer-reviewed evidence that AAV-delivered secreted Klotho extends mouse lifespan, and FDA orphan designation plus manufacturing kickoff for an ALS gene therapy. Here is how Klotho could translate from bench to bedside.
The longevity gene finally gets a clinical runway
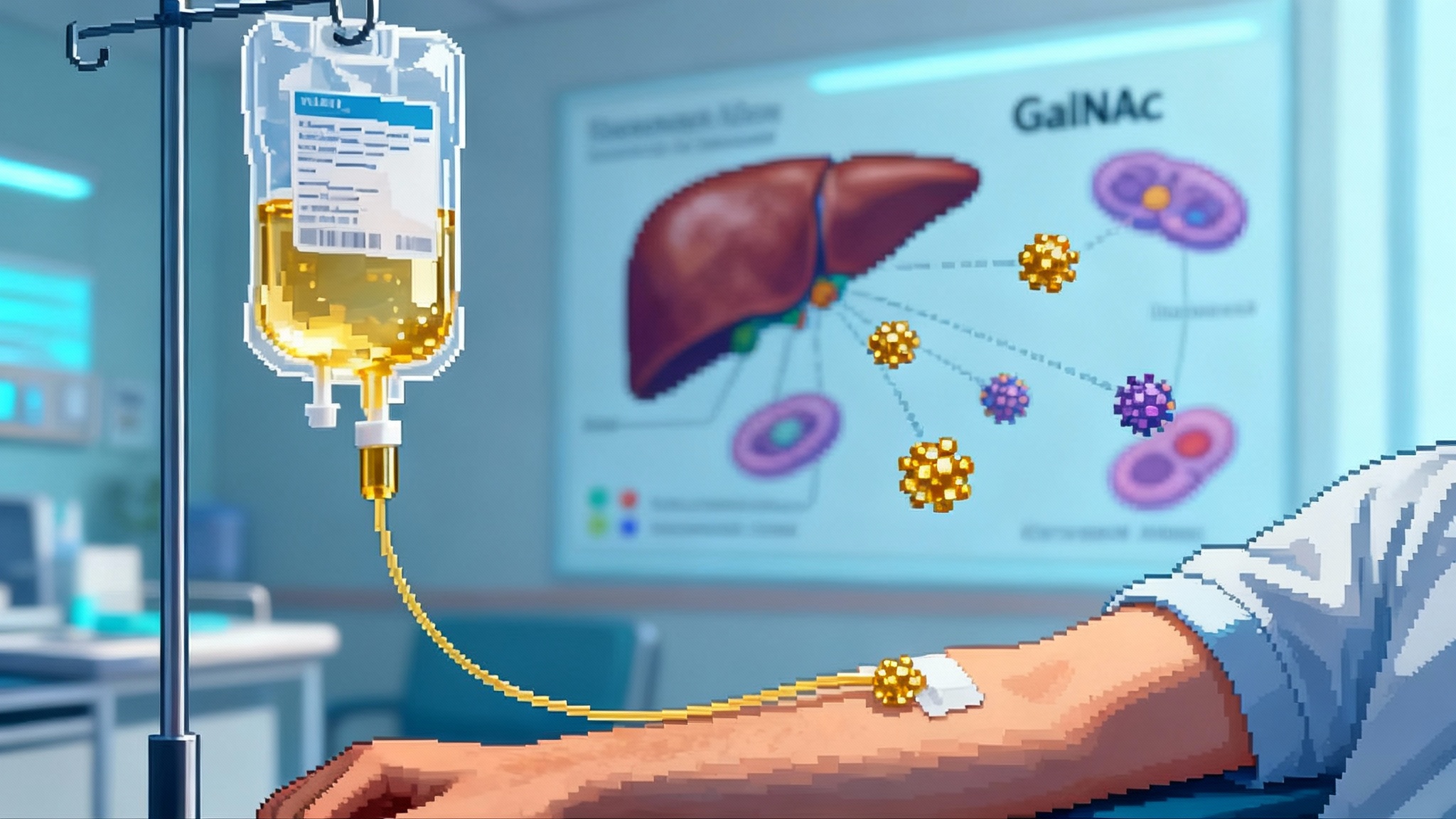
In 1997, researchers discovered a gene that seemed to slow the tick of biological time in mice. It was called Klotho, and for more than two decades it was the stuff of conference slides and animal studies. In 2025, the story changed. A peer-reviewed study in Molecular Therapy reported that delivering the secreted form of Klotho with an adeno-associated virus boosted lifespan in male mice by roughly 15 to 20 percent and improved muscle, bone, and brain aging markers. The team turned the body into a factory for a protective protein rather than dripping in a drug from the outside. See the s-Klotho lifespan study in mice.
Weeks later, the first clinical chess pieces moved. On July 10, 2025, Klotho Neurosciences announced that the United States Food and Drug Administration had granted Orphan Drug Designation for KLTO-202, an adeno-associated virus therapy designed to drive expression of secreted Klotho for amyotrophic lateral sclerosis. The company also said it was initiating manufacturing and regulatory preparations. Read the announcement: FDA orphan designation for KLTO-202.
The combined effect of these milestones is straightforward. A venerable longevity gene moved from extending the lives of mice in a controlled lab to the threshold of human testing. That mirrors the broader momentum we tracked in the cross-organ trial pivot in 2025 and complements lessons from the first human longevity trial lessons.
What makes Klotho different
Klotho comes in two main forms. Membrane Klotho sits on cells, especially in the kidney, where it helps regulate phosphate and vitamin D through fibroblast growth factor 23. Secreted Klotho circulates in the blood and acts like a systemic caretaker. In plain terms, membrane Klotho is like a thermostat for mineral balance, while secreted Klotho is like a maintenance crew that reduces wear and tear across multiple rooms in the house.
This maintenance role matters because aging is rarely a single-room problem. Sarcopenia weakens muscle, osteoporosis thins bone, inflammation fogs the brain, and immune function becomes erratic. A therapy that raises secreted Klotho has the potential to touch several of these systems at once. That is why the 2025 mouse data drew so much attention. The benefit was not limited to one tissue. Investigators reported better muscle regeneration, less muscle fibrosis, improved bone microstructure, and signals of enhanced neurogenesis. When a single intervention nudges multiple age-linked measures in the same direction, the clinical imagination starts to work.
From mouse to manufacturing to first-in-human
Translating an aging target to a human trial is uphill work. Klotho Neurosciences is taking a practical route. The company’s lead program aims at a specific disease, amyotrophic lateral sclerosis, where patients face a rapid decline and new options are urgently needed. The strategy is to target expression of secreted Klotho primarily in muscle using a muscle-specific promoter. The rationale is straightforward. Muscle is abundant and vascular, so it can serve as a biofactory that secretes Klotho into the bloodstream. In theory, that spreads the protein to the neuromuscular junctions and possibly the central nervous system, without forcing extremely high doses that concentrate in the liver.
On the operations side, 2025 brought visible progress. The company announced it was moving into process development and manufacturing and later struck a manufacturing agreement to use a next-generation adeno-associated virus platform. Those are not cosmetic updates. For gene therapy, the factory is as critical as the formula. Yields, impurities, and batch-to-batch consistency decide whether a candidate ever reaches patients. The signal to watch through late 2025 and early 2026 is whether the team completes the Chemistry, Manufacturing, and Controls package and preclinical safety studies on time. Without those, there is no Investigational New Drug submission and no Phase 1.
How a first human trial might look
Expect a traditional dose-escalation Phase 1 or Phase 1/2 design in amyotrophic lateral sclerosis. The most likely plan includes:
- Population: Adults with early amyotrophic lateral sclerosis or fast-progressing disease, screened for preexisting neutralizing antibodies to the chosen adeno-associated virus serotype. Early disease increases the chance to detect functional benefit and avoids treating end-stage patients who may not tolerate immune suppression.
- Delivery and dosing: A single intravenous infusion at rising dose levels. Some programs also explore intrathecal dosing to achieve central nervous system exposure at lower systemic doses. A muscle-directed promoter aims to favor expression in muscle to create a systemic Klotho source.
- Background therapy: Standard of care allowed. Concomitant steroids or other immune modulators often accompany adeno-associated virus dosing to blunt inflammatory reactions.
- Primary endpoints: Safety and tolerability, including infusion reactions, liver enzymes, complement activation markers, and organ function. Gene therapy programs live or die on safety in the first few cohorts.
- Key pharmacology: Serum secreted Klotho levels over time, vector genome copies in blood and possibly in muscle biopsies, and anti-capsid and anti-Klotho immune responses. These readouts answer whether the body is making the protein and whether immunity threatens durability.
- Function and disease progression: Change in the slope of the ALS Functional Rating Scale Revised, respiratory capacity such as slow vital capacity or forced vital capacity, quantitative muscle testing, and survival or time to tracheostomy as longer-term outcomes. Biomarkers such as neurofilament light chain give early signals on neurodegeneration.
Importantly, even if the first signal is restricted to disease progression, a clean safety and pharmacology profile would open the door to broader aging indications where Klotho’s multi-tissue effects could matter.
Endpoints beyond neurology, and how to measure them
If secreted Klotho is truly a systemic maintenance crew, trials will eventually need to prove benefits in muscle, bone, brain, and perhaps immune aging. Here is what a broader development plan could include:
- Sarcopenia and frailty: Objective measures such as gait speed, the Short Physical Performance Battery, the 6 minute walk test, stair-climb power, and isokinetic dynamometry. Muscle biopsies could quantify fiber size and fibrosis. Wearables can capture daily step patterns and gait variability at home.
- Bone aging: Dual energy X ray absorptiometry for bone mineral density, high resolution peripheral quantitative computed tomography for microarchitecture, and the rate of new fragility fractures in longer studies. Serum phosphate, calcium, parathyroid hormone, vitamin D, and fibroblast growth factor 23 must be monitored because Klotho intersects mineral metabolism.
- Brain aging: Composite cognitive batteries for processing speed and executive function, digital speech and handwriting analysis for early decline, and magnetic resonance imaging markers such as hippocampal volume. Cerebrospinal fluid or plasma neurofilament light can serve as a general neurodegeneration marker.
- Immune aging: Vaccine response titers, T cell receptor diversity, and inflammatory cytokines. Secreted Klotho has been reported to reduce inflammatory signaling in preclinical work, so immune readouts can help connect mechanism to function. This links to the broader regulatory context in reboots the aging biomarker race.
- Biological age: Epigenetic clocks are not yet accepted regulatory endpoints, but they can be supportive evidence. If Klotho meaningfully slows functional decline, a coherent shift in biological age estimates would strengthen the narrative.
The point is not to chase every measure at once. It is to pick two or three systems where the biology is strongest and run convincing, adequately powered studies that regulators and clinicians will trust.
The engineering puzzle: how to deliver Klotho safely
Gene therapy delivers blueprints, not proteins. Adeno-associated virus vectors are popular because they do not replicate and can persist for years in non dividing cells. They also come with real constraints.
- Tropism and dose: Adeno-associated virus serotype 9 favors the liver after intravenous dosing. That is one reason programs lean on tissue specific promoters to bias expression and on careful dosing to avoid liver injury. Muscle targeting makes sense for a secreted protein, but intravenous delivery can still drive liver exposure. Careful steroid prophylaxis and dose selection are essential.
- Immunity: Many adults have preexisting antibodies to adeno-associated virus capsids, which can exclude them from trials or blunt efficacy. Infusion can also trigger complement activation, which has been linked to rare but serious events in high dose gene therapy. Ramping dose slowly, using human compatible promoters, and avoiding extreme vector loads are part of the risk plan.
- Durability and control: A single dose that lasts for years is attractive, but not if expression drifts too low or too high. Too little and the effect fades, too much and phosphate metabolism could tilt toward hypophosphatemia. Protocols should include long follow up, with safety labs for phosphate and vitamin D and a plan to manage overexpression if it occurs.
- Manufacturing: Old triple transfection methods strain to produce consistent, high titer vectors at scale. Newer producer cell lines and helper plasmid systems can improve potency and purity. Without that, timelines slip and batches fail release testing.
Because Klotho is secreted, there are alternatives that sidestep some of these issues. Protein therapy could deliver recombinant Klotho directly, offering fine dose control and easy stoppage if adverse events surface. Messenger RNA formulations could program transient protein production with repeat dosing. Each path trades convenience and control for durability and manufacturing complexity. Expect at least one messenger RNA player to test a Klotho approach in healthy older adults as a dose ranging safety study before moving into disease populations.
Why ALS first does not mean ALS only
Using amyotrophic lateral sclerosis as the entry point is a strategic choice. The disease has clear unmet need, strong patient advocacy, and a regulatory framework that understands neurodegeneration. A therapy that shows a dose dependent rise in secreted Klotho levels and an acceptable safety profile could move rapidly into a randomized Phase 2 to test function, with survival measured over longer follow up.
If those data trend positive, expansion cohorts can aim at other motor neuron diseases or muscular dystrophies that share neuromuscular junction pathology. Meanwhile, exploratory studies in sarcopenia or osteoporosis could recruit older adults without neurodegenerative disease and use functional and imaging endpoints that read out within 6 to 12 months. The broad appeal of Klotho is that success in one system does not preclude success in another. A single promoter driven muscle biofactory could, in theory, feed multiple organs.
Fast followers and how this market could form
The first wave of Klotho therapeutics will likely include three modalities:
- Adeno-associated virus gene therapy: Long acting, single dose, with muscle directed expression of secreted Klotho. High upfront risk, high potential durability. Attractive for diseases where one and done is valuable and close monitoring is feasible.
- Messenger RNA delivery: Repeat dosing in lipid nanoparticles that induce temporary Klotho production. More controllable, easier to dial back or stop, and potentially better suited for prevention or early intervention studies in older adults.
- Recombinant protein: Direct infusions of Klotho or stabilized fragments. Immediate control over exposure, but shorter half life and a harder time reaching the brain. Still attractive for proof of mechanism in muscle or bone endpoints.
Expect companies to differentiate by delivery tissue, dose control, and patient selection. One may focus on neuromuscular junction disorders, another on sarcopenia with falls as a clinical endpoint, and another on cognitive aging with digital biomarkers and cerebrospinal fluid readouts. The shared thread will be serum Klotho levels and a menu of organ specific outcomes that prove real-world benefit.
What to watch between now and first dosing
- Regulatory interactions: A successful pre-Investigational New Drug meeting will telegraph that the toxicology, biodistribution, and Chemistry, Manufacturing, and Controls plans match regulator expectations. If new immune risk mitigation is required, expect timelines to move.
- Manufacturing milestones: Look for updates that lots have been released to clinical specifications, not just that a partner has been signed. That is the difference between a press release and a vial on ice.
- Early human pharmacology: When first-in-human studies begin, the signal to watch is a clear, dose related rise in serum secreted Klotho. Without that, even the best functional endpoint will be ambiguous.
- Choice of controls: Many gene therapy trials start open label for safety. The sooner randomization and masking arrive, the sooner the field can distinguish real effects from regression to the mean.
- Parallel programs: Messenger RNA and protein approaches that raise Klotho in a graded fashion could validate target engagement in healthy older adults with quick readouts like gait speed or the 6 minute walk test. That would complement the disease focused adeno-associated virus path.
The grounded upside
There is a temptation to declare each longevity result a moonshot. Klotho does not need that. It needs careful dosing, sober trial design, and endpoints that regulators and clinicians consider meaningful. The 2025 Molecular Therapy paper suggests that Klotho is not just another lab curiosity. The orphan designation and manufacturing kickoff show that at least one team is treating it like a drug candidate with a path to patients.
If the first human studies confirm safety and show sustained increases in secreted Klotho, the field will have a credible geroscience intervention in play. The next steps are practical. Choose the systems where Klotho should matter most, pick endpoints that move within a year, and keep the engineering simple enough to execute. Aging is a complex house with many rooms, but sometimes the right maintenance crew can make the whole place feel new again.