Longevity
Longevity
Articles under the Longevity category.
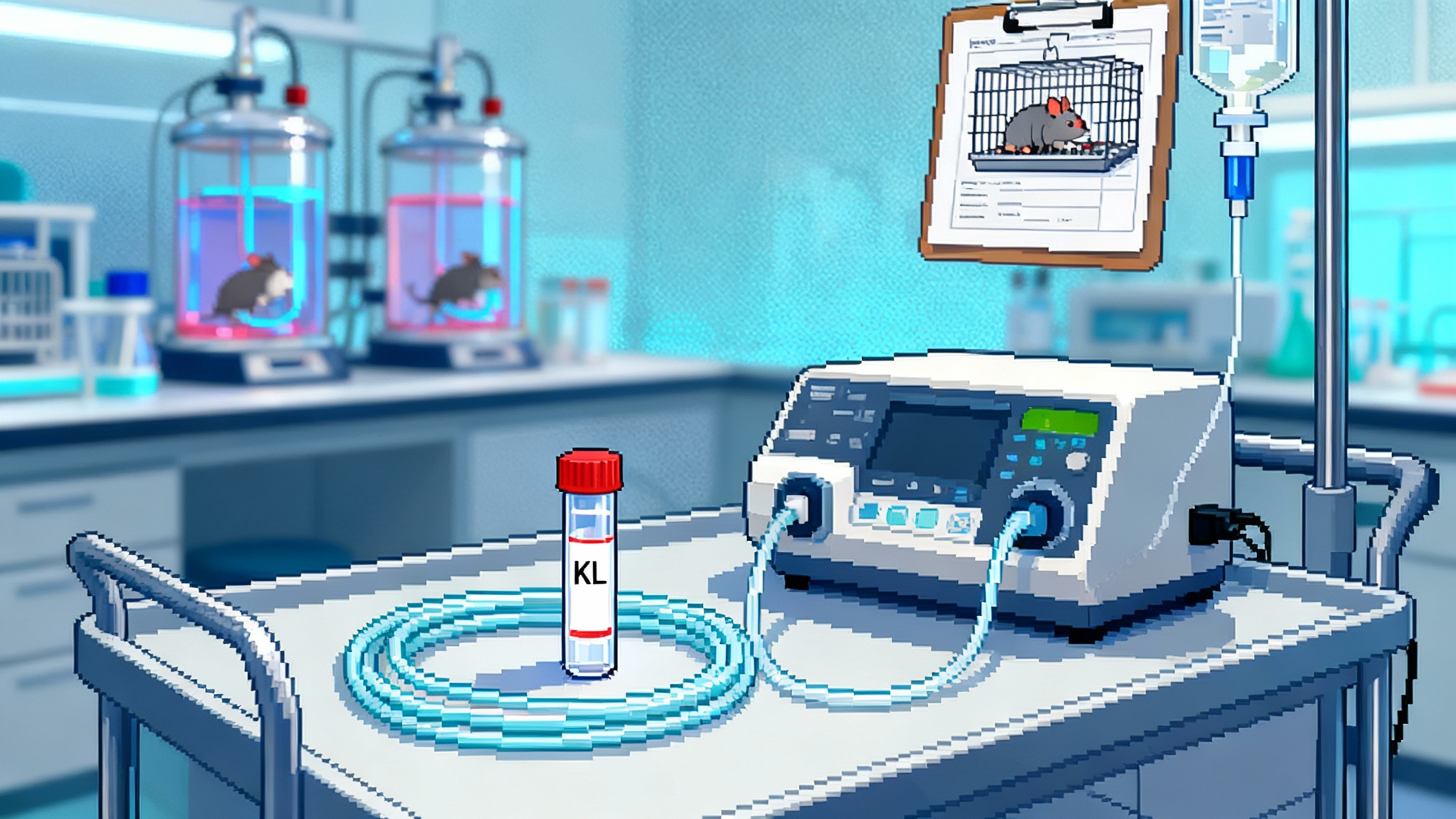
Klotho Goes Clinical: From Mouse Longevity to Human Trials
Two 2025 milestones moved the classic longevity gene into the clinic: peer-reviewed evidence that AAV-delivered secreted Klotho extends mouse lifespan, and FDA orphan designation plus manufacturing kickoff for an ALS gene therapy. Here is how Klotho could translate from bench to bedside.
PEARL lands: decoding rapamycin’s first human longevity trial
PEARL just delivered the first randomized human data on weekly rapamycin for aging. Safety looked acceptable, the primary endpoint did not move, and one sex-specific signal stood out. Here is what the study means now and the fastest path to an actual label with outcomes that matter.
In vivo CAR-T goes clinical: unlocking senescence therapy
September 2025 delivered two thresholds for cell therapy: the first human data that T cells can be programmed into CAR T in the body without conditioning, and the first randomized win for CAR T in a solid tumor. Together they open a credible path to senescent cell clearance in common aging diseases.
Mitochondria’s Breakthrough Year: Rare Cures to Longevity
Summer 2025 delivered three milestones for mitochondrial medicine: births after mitochondrial donation in the UK, an FDA-cleared Phase II cell therapy for Pearson syndrome, and precise base edits in animals and patient cells. Here is what is real, what is risky, and how this momentum could shape healthspan.
Dogs-first longevity passes a real FDA test at Loyal
Loyal’s senior-dog pill just passed the FDA’s Reasonable Expectation of Effectiveness bar, opening a path to conditional approval. Here is what RXE certifies, why the veterinary-first route matters, and how a 2026 launch could become real.
FDA’s LDT reversal reboots the aging biomarker race
With the FDA’s 2024 LDT rule vacated and CLIA back in the driver’s seat, aging biomarkers from methylation clocks to proteomic panels are racing into clinics. Here is what reopens, who benefits now, and a 6 to 12 month playbook to do it credibly.
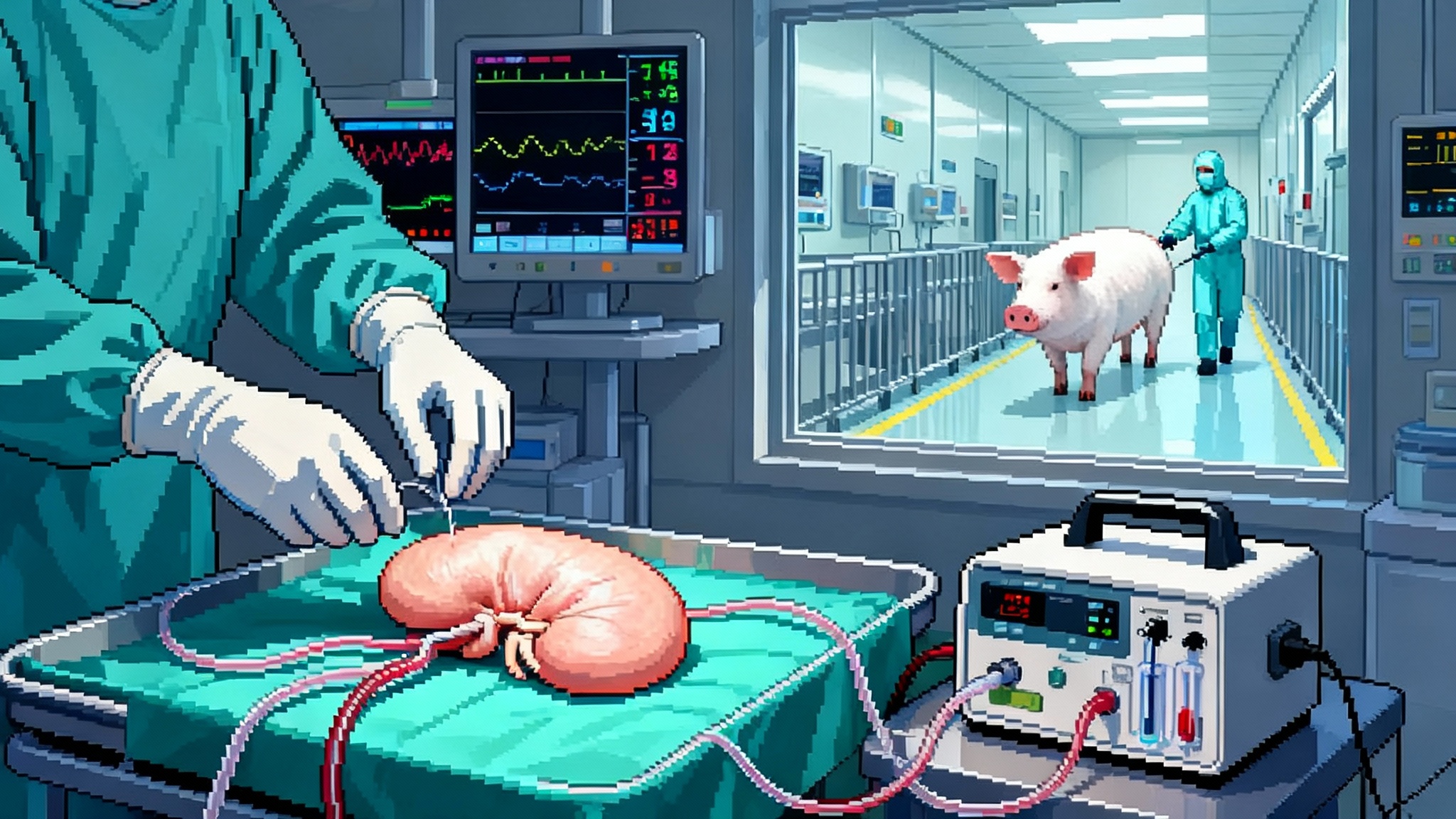
Pig Kidneys Enter Trials: 2025’s Biggest Longevity Bet
For the first time, U.S. regulators have authorized formal studies of gene-edited pig kidneys. Here is what changes for patients, safety, and longevity if xenotransplantation moves from one-off surgeries to scalable care.
Taurine’s reality check: NIH study drops biomarker hype
An NIH analysis in Science on June 5, 2025 reports that circulating taurine does not consistently track aging in humans, monkeys, or mice. We explain biomarker versus endpoint, where human trials stand, and how to read longevity claims with discipline.
GLP-1s, Healthspan, and a 6.4% Mortality Reset by 2045
Swiss Re modeling suggests GLP-1 drugs could reduce U.S. mortality up to 6.4 percent by 2045. With new Phase 3 data on oral semaglutide, we unpack biology, durability, biomarkers, trials, payer models, and equitable access.
Cross-organ PER data mark 2025 pivot to first human trials
Cross-organ signals from Life Biosciences at ARDD 2025 move partial epigenetic reprogramming from hype to clinical planning. Here is what it means for delivery, safety, endpoints, first indications, and regulatory strategy.
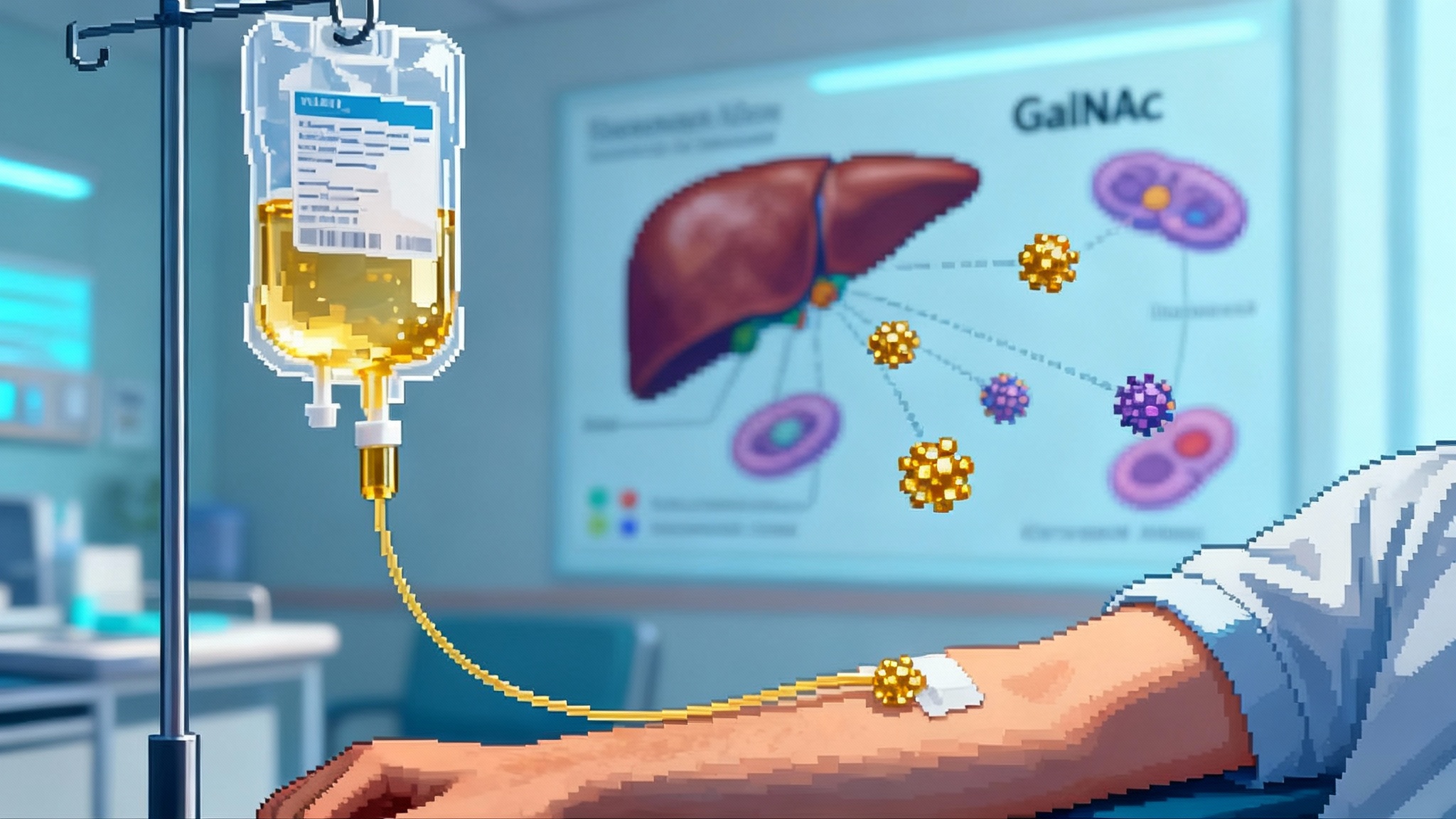
Lilly’s Verve buy puts one-shot LDL editing on the longevity map
Lilly’s July 2025 purchase of Verve puts one-shot PCSK9 base editing on a fast track from lab signal to real-world prevention. Here is what the data showed, the risks, the regulatory path, and what to watch next.
GPX4 Senolytics Hit Clinic: Rubedo’s RLS‑1496 Moves Into Humans
Rubedo dosed its first patient with RLS-1496 in May 2025, launching the first human test of a GPX4-modulating senolytic. Here is how ferroptosis could define senolytics 2.0, which skin biomarkers will matter, and what a credible 2026 systemic program could look like.
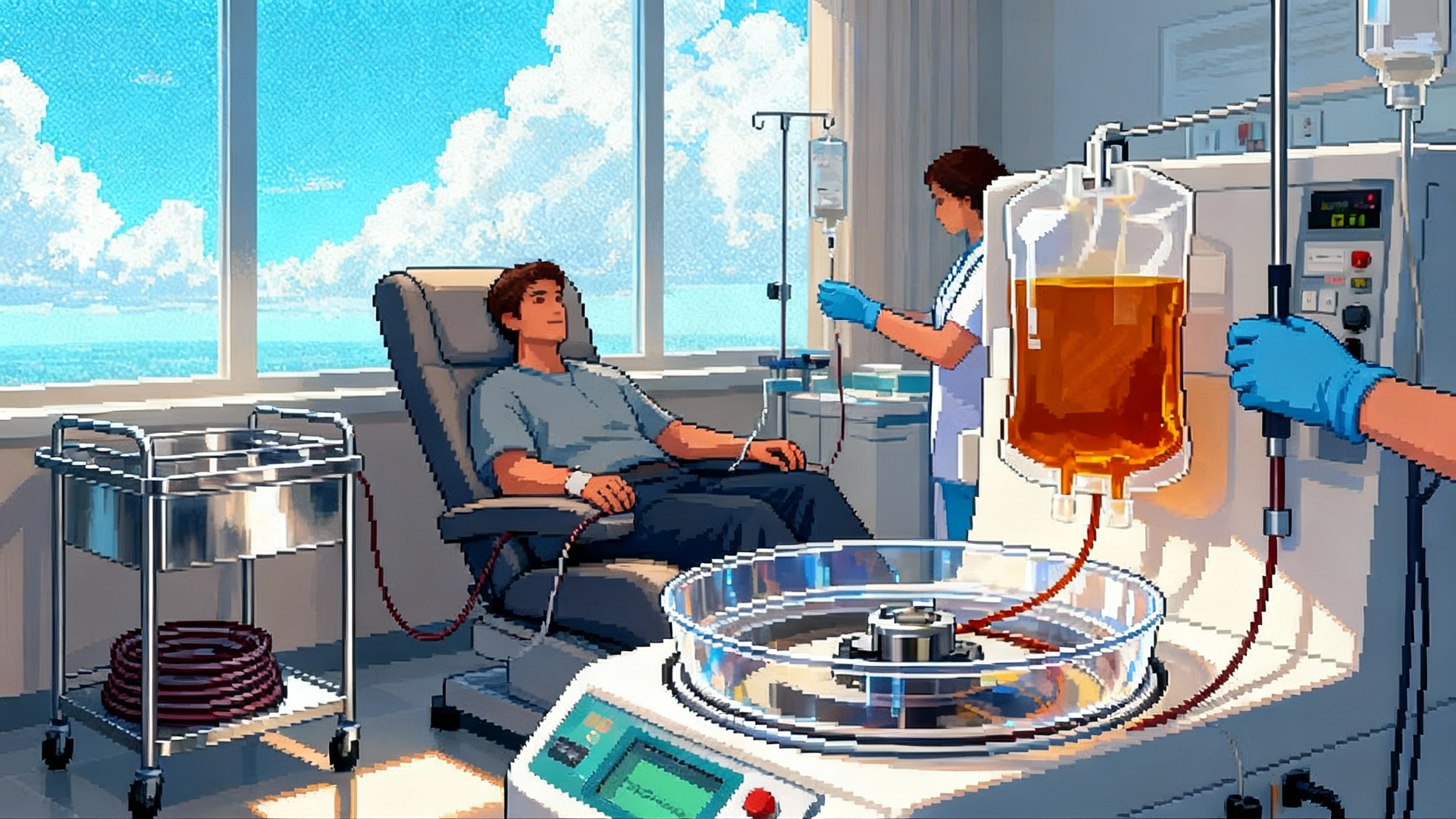
Plasma Exchange’s 2025 Pivot: Human Data and Expansion
A randomized human study in 2025 reported multi-omics rejuvenation signals after therapeutic plasma exchange, strongest when IVIG was added. Here is what the data means, how clinics plan to scale, and what the next randomized trials must prove.
PEARL’s year-long rapamycin trial: what it really shows
PEARL's 48-week randomized trial in healthy adults reports acceptable safety, sex-specific gains in lean mass, and less pain in women, with no change in visceral fat. Here is what that means for healthspan and what a real Phase 3 must prove.
Retro Bio’s autophagy Alzheimer’s pill nears first human test
{"value":"Retro Biosciences plans to dose its brain-targeted autophagy inducer, RTR242, in a 2025 first-in-human study in Australia. Here is what the trial will likely test, which biomarkers matter most, and how this approach stacks up against rapalogs and supplements."}
Canine longevity hits clinics: Loyal’s RXE and the TRIAD reboot
Veterinary anti-aging just crossed a regulatory threshold. The FDA’s CVM granted RXE to Loyal’s LOY‑002 while the Dog Aging Project restarted its TRIAD rapamycin trial, signaling that geroscience is moving into everyday practice.
From longer-lived mice to humans: Klotho therapy’s 2025 pivot
A single AAV dose of secreted Klotho extended male mouse lifespan in a 2025 study, while a biotech began manufacturing an AAV‑Klotho candidate for ALS. Here is the biology, delivery tradeoffs, safety map, endpoints, and timelines that matter now.
What a 117-year-old taught us about aging and disease
A 2025 case study of supercentenarian Maria Branyas Morera reveals low inflammation, a youthful microbiome, protective genes, and a younger epigenome. The findings shift longevity science toward resilience and trial designs that measure recovery and function, not just age.