Longevity
Longevity
Articles under the Longevity category.
FDA LDT rollback: how longevity biomarkers return to clinics
After a March 31, 2025 court ruling, FDA formally rescinded its 2024 LDT rule on September 19, 2025. Epigenetic clocks and proteomic biological age panels shift back under CLIA. Here is what is defensible now, how to validate, and what to watch next.
Colchicine vs Clonal Aging: Slowing TET2-Driven Mutations
Fresh August 2025 cardiology genetics data suggest that low-dose colchicine may slow TET2-driven clonal hematopoiesis in people with coronary disease. Here is what CHIP is, who to test, and how to act while we wait for genotype-enriched trials.
The GLP-1 Muscle-Sparing Race to Real Longevity Gains
New 2025 data suggest GLP-1 combinations can shift weight loss toward fat while protecting muscle. Here is how ActRII and myostatin strategies, amylin add-ons, and smarter training could make weight loss longevity-safe for older adults.
Taurine hype, meet reality: NIH’s 2025 Science verdict
A June 5, 2025 analysis from NIH in Science reports that circulating taurine does not consistently decline with age, challenging taurine’s status as an aging biomarker. Here is what that means for supplements, clinical trials, and how to judge real biomarkers.
Senolytics at St. Jude: frailty trial and aging vaccines
In August 2025, St. Jude’s SEN-SURVIVORS trial moved to active not recruiting, testing dasatinib plus quercetin against fisetin to boost walking speed and lower T‑cell p16INK4a in adult childhood‑cancer survivors. If the biomarker and function both shift, it could lay the groundwork for precision aging vaccines.
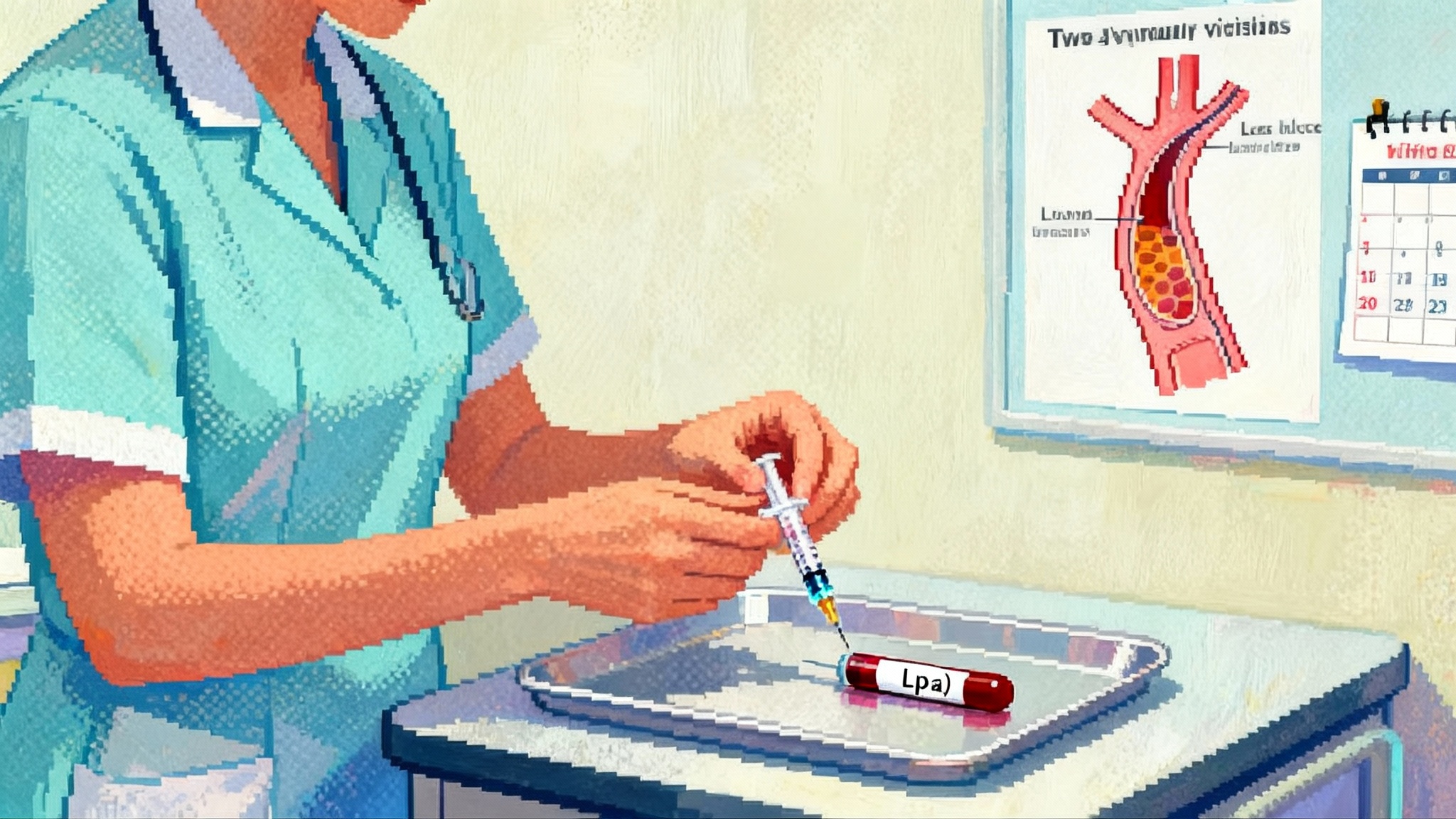
Lp(a) goes mainstream: RNA shots reset lifelong heart risk
In March 2025, lepodisiran delivered striking and durable Lp(a) reductions, while pelacarsen’s first outcomes readout is guided for 2026. Here is why Lp(a) is causal, why lifestyle will not fix it, and how rare dosing could reshape midlife heart risk.
Alzheimer’s enters the bloodstream: pTau217 tests arrive
From the May 16, 2025 FDA clearance to an August nationwide rollout, a plasma pTau217 blood test is reshaping how we detect Alzheimer’s pathology. See who should be tested, how to interpret results, and what this means for care, prevention, and access.
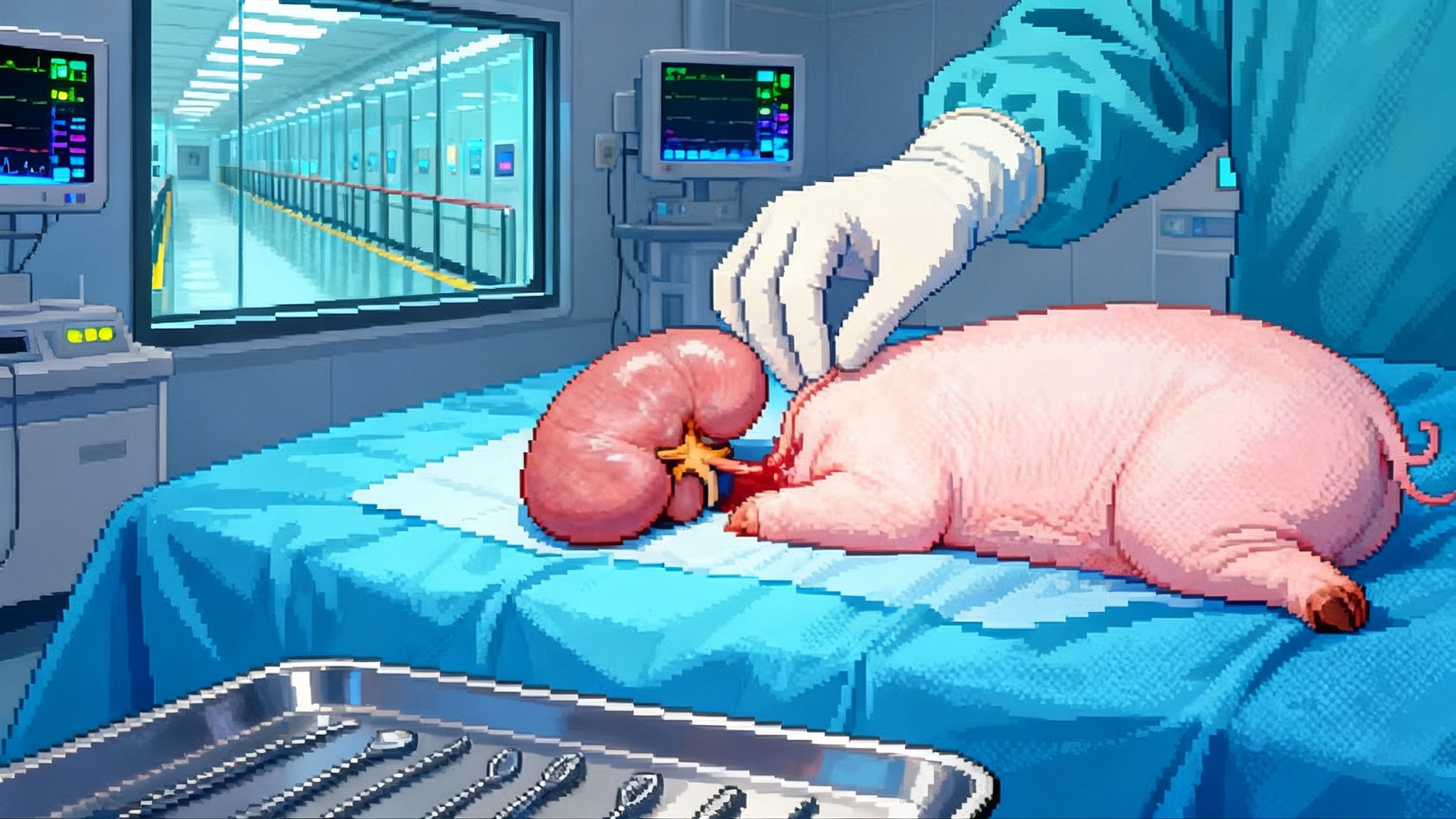
2025’s Turning Point: Gene-Edited Pig Kidneys in Trials
2025 marks a clinical turning point for xenotransplantation. With two FDA-cleared trials for gene-edited pig kidneys, multiple compassionate-use surgeries, and a first study of external pig-liver support, the field shifts from headlines to care pathways.
Thymus Rejuvenation 2025: Restoring Immune Youth at the Source
2025 is the year thymus repair moves from speculation to serious clinical plans. New mechanisms, early human signals, and active programs converge on one goal: restore thymic function to refresh naive T cells, strengthen vaccine responses, cut infections, and extend healthspan.
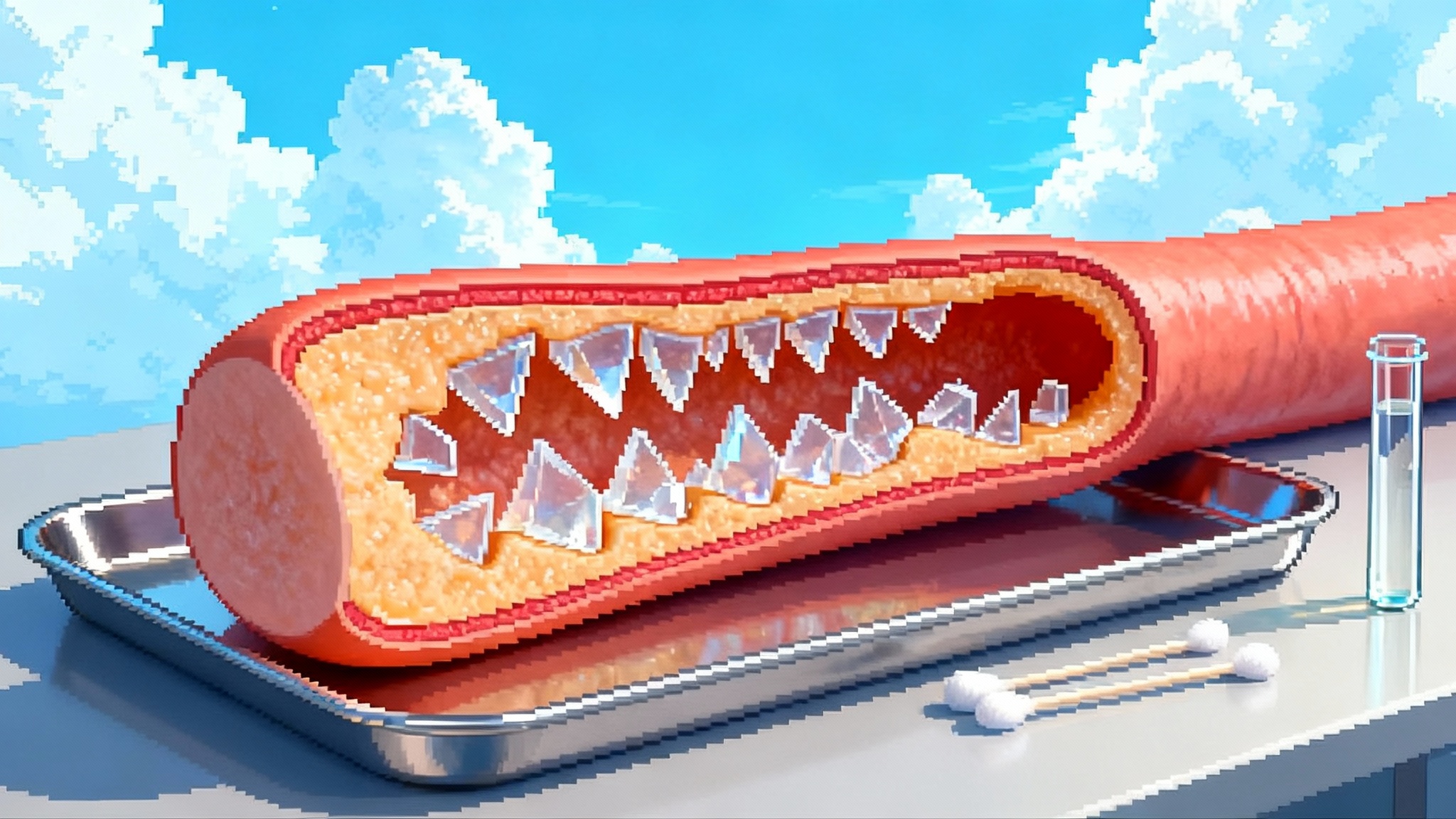
Tiny plastics, aging arteries, and your heart risk
New data from 2024 and spring 2025 detect micro and nanoplastics inside human carotid plaques and link them to higher rates of heart attack and stroke. Learn what this means for vascular aging and the practical steps you can take to reduce exposure today.
Lilly’s Verve buy puts one-shot PCSK9 editing in play
Eli Lilly’s July 2025 purchase of Verve Therapeutics moves single-dose PCSK9 base editing from bold concept to near-term clinic. If durable, one infusion that halves LDL could compress decades of cardiovascular morbidity and redefine preventive cardiology.
Partial Reprogramming Hits the Primate to Human Pivot
Late August 2025 data from Life Biosciences show inducible OSK gene therapy restoring function and youthful epigenetic signatures in nonhuman primate optic nerve injury models. Here is what it means, the safety questions ahead, and what to watch before first-in-human studies in early 2026.
FDA backs a dog longevity pill, a first for lifespan claims
On February 26, 2025 the FDA’s Center for Veterinary Medicine accepted Loyal’s efficacy package for LOY-002, a daily pill for senior dogs. Here is why that first-of-its-kind signal matters, how conditional approval works, and what to watch next.
GLP-1s at Scale: From Weight Loss to Longer, Stronger Lives
September 2025 delivered two signals that reset the GLP-1 narrative: Swiss Re modeled population-level mortality gains, and new oral semaglutide data showed injectable-level weight loss. Here is how to translate weight loss into real healthspan while protecting muscle and bone.
FDA LDT Rollback Resets the Longevity Biomarker Market
A 2025 court decision and FDA rescission return LDTs to CLIA oversight. Epigenetic clocks and proteomic biological age panels are reentering clinics. Here is what changes for validation, safety, and reimbursement.
Autophagy in the clinic: Retro's pill targets brain aging
Retro Biosciences plans to begin first-in-human testing of RTR242, an autophagy-boosting oral drug aimed at Alzheimer’s, by the end of 2025. Here is how cellular cleanup could translate into healthspan gains, the endpoints that matter, and the regulatory pathway most likely to deliver approval.
Rome 2025: Senolytics Move From Hype to Clinical Plans
Post-event analysis of the September 16 to 19, 2025 ICSA and Phaedon Senotherapeutics Summit in Rome. New human data, clearer biomarker roadmaps, and a push for assay harmonization point to how senolytics could reach the clinic in the next 12 to 24 months.
Autophagy’s Big Test: A Brain‑Aging Pill Hits the Clinic
A first-in-human Alzheimer’s study of an oral autophagy enhancer is about to test whether restoring cellular clean-up can slow neurodegeneration. Here is why proteostasis matters, what to watch in the trial, and what a readout could unlock next.