Autophagy’s Big Test: A Brain‑Aging Pill Hits the Clinic
A first-in-human Alzheimer’s study of an oral autophagy enhancer is about to test whether restoring cellular clean-up can slow neurodegeneration. Here is why proteostasis matters, what to watch in the trial, and what a readout could unlock next.
The news: autophagy moves from theory to people
In September 2025, Retro Biosciences announced its first human study of RTR242, an oral small molecule designed to boost autophagic flux. First dosing is planned in Australia before year end, with Alzheimer’s disease as the opening indication. The company frames the drug as a way to revive the brain’s recycling system and potentially reverse aspects of brain aging. That makes this trial an early real-world test of a major longevity idea in a highly visible disease space. See reporting on the Retro Biosciences first human trial.
This is not another anti-amyloid antibody story. It is a bet that restoring proteostasis inside neurons and glia can move the needle on cognition and function. If that premise holds up in people, it could open the door for autophagy-centric therapeutics across multiple age-related diseases.
Why proteostasis is central to longevity biology
Longevity science keeps returning to a core theme: healthy cells continuously repair, recycle, and renew. The word for that internal housekeeping is proteostasis, a dynamic balance between protein synthesis, folding, trafficking, and degradation. Break that balance and damaged proteins accumulate, organelles falter, and stress signaling ramps up. Across organisms, interventions that preserve proteostasis track with longer healthspan. For foundational background, see our primer on autophagy basics for longevity.
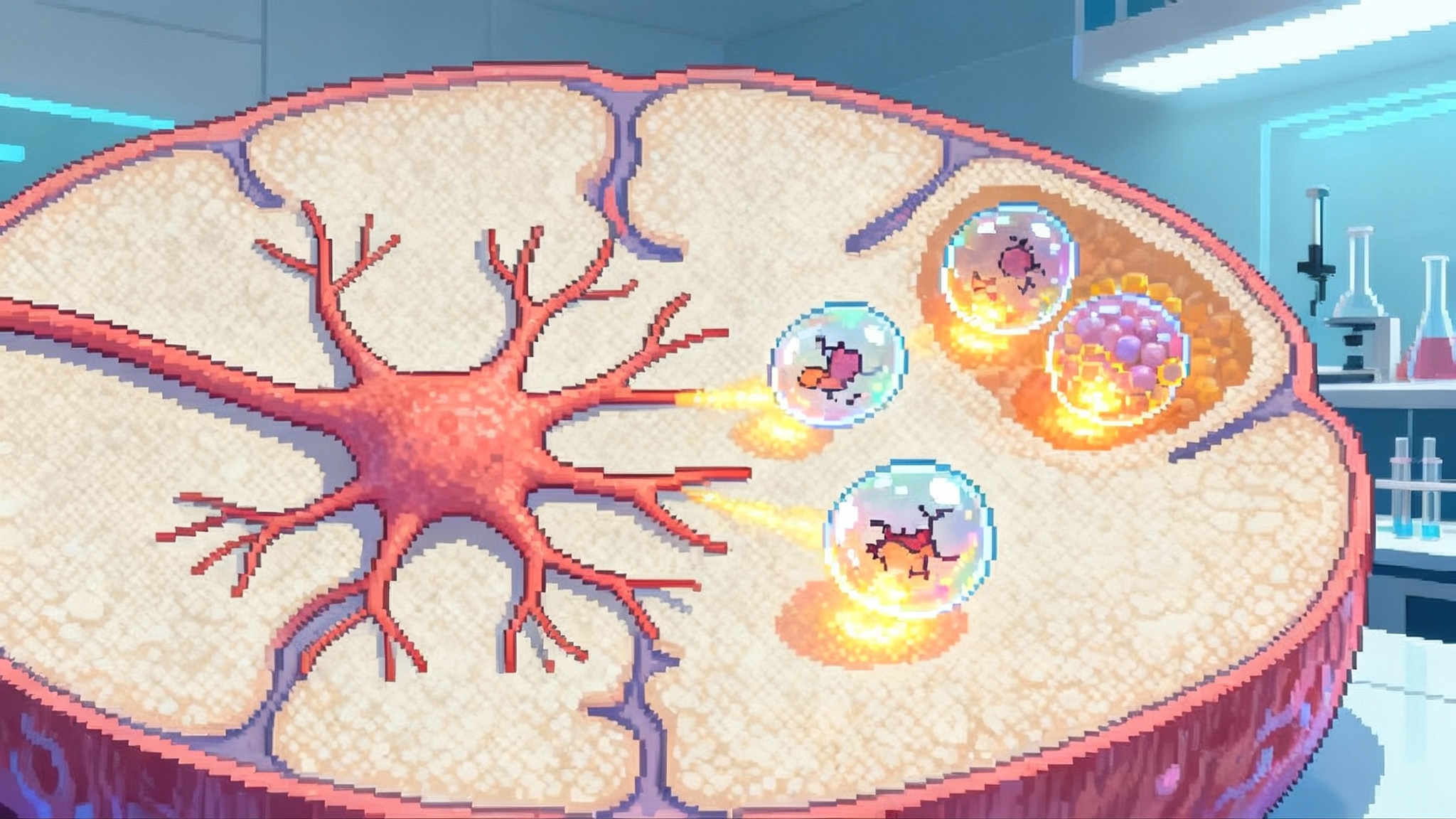
Autophagy is the most versatile arm of the clean-up crew. It engulfs damaged proteins and organelles in double-membrane vesicles, then fuses with lysosomes where cargo is degraded and recycled. Autophagy is tuned by nutrient-sensing pathways. When nutrients are abundant, mTORC1 activity suppresses autophagy and promotes growth. When nutrients are scarce or cells face stress, AMPK and related sensors lift that brake and autophagy clears debris. Over decades, the system loses efficiency. Cargo selection becomes erratic, lysosomes become less acidic, and trafficking slows, so garbage piles up and cells become vulnerable.
The idea behind an autophagy-boosting drug is straightforward. If aging blunts the clean-up machinery, then nudging it back toward youthful throughput could stabilize neurons, improve their bioenergetic resilience, and reduce the toxic feedback loops that drive neurodegeneration.
What autophagy is, and how it falters with age
Autophagy proceeds in stages. Cells build a cup-shaped phagophore, elongate it to an autophagosome that sequesters cargo, then fuse with lysosomes to create an autolysosome where enzymes degrade the contents. Selective autophagy uses receptors like p62 and NBR1 to tag aggregates or organelles. In neurons, which must last a lifetime and cannot dilute damage by cell division, autophagy is indispensable. Reviews across model organisms and human tissues describe the pattern: autophagic flux declines with age, and restoring it is associated with better healthspan. For mechanisms and context, see the Annual Reviews overview on the diverse cellular roles of autophagy.
One important nuance is that simply making more autophagosomes is not the goal. Flux matters. If lysosomes are not working, pushing cargo into the system can cause a traffic jam. The right drug should enhance throughput from initiation to degradation without overwhelming a compromised endpoint. That is why pharmacodynamic biomarkers that reflect real flux are so important in first-in-human studies.
Why start with Alzheimer’s as the wedge
If autophagy sits upstream of many age-related pathologies, why pick Alzheimer’s first? Three strategic reasons stand out:
-
Clear unmet need and visible endpoints. Even with recent antibody approvals, many patients need safer, easier therapies. Trials can read out on cognitive batteries and fluid biomarkers that regulators and clinicians already use. For an orientation to these assays, see our Alzheimer’s biomarker guide.
-
Biology that fits the mechanism. The Alzheimer’s brain contains misfolded proteins, stressed mitochondria, and dysfunctional lysosomes. Enhancing autophagic flux could relieve multiple insults at once, not just one species of plaque or tangle.
-
A proving ground for geroscience. If a drug that restores cellular clean-up improves signs of neurodegeneration in humans, the same principle could extend to Parkinson’s, frontotemporal dementia, and perhaps non-neurologic indications where proteostasis erodes with age.
There is also a practical angle. Australia provides a faster on-ramp for first-in-human safety studies. A clean Phase 1 signal on safety, CNS penetration, and target engagement would justify larger, longer efficacy trials in Alzheimer’s or in earlier cognitive aging.
What to measure: biomarkers and outcomes that matter
Early studies must establish more than basic safety. They must show that the drug gets into the brain, engages its target, and nudges the biology in the intended direction. For an autophagy-boosting Alzheimer’s program, the most telling readouts cluster into six buckets.
- Pharmacokinetics and CNS exposure
- Plasma and CSF drug levels to confirm brain penetration.
- Dose proportionality and half-life estimates to pick rational regimens.
- Target engagement and autophagy flux
- Peripheral pharmacodynamics in blood cells, for example changes in p62 and LC3 lipidation patterns ex vivo after stimulation, interpreted cautiously since blood is not brain.
- Lysosomal function markers, such as cathepsin activity assays or TFEB target gene expression signatures in peripheral cells, to suggest downstream activation.
- If available in CSF, exploratory proteomic panels enriched for autophagy and lysosome proteins to detect pathway shifts.
- Core Alzheimer’s fluid biomarkers
- CSF or plasma phosphorylated tau (pTau217 or pTau181) and Aβ42/40 ratio to see if upstream clean-up indirectly reduces production or enhances clearance of pathological species.
- Neurofilament light chain for axonal injury. Stabilization or slowing of rise over months would be meaningful.
- GFAP and sTREM2 to track astrocytic and microglial reactivity, since improved proteostasis could modulate neuroinflammation.
- Imaging
- Structural MRI for hippocampal and cortical atrophy rates. A slower rate than expected over 6 to 12 months would be encouraging.
- Tau PET and amyloid PET are not required for a non-amyloid mechanism, but changes would strengthen a disease-modifying narrative if present.
- FDG-PET or synaptic density PET as optional signals of functional rescue, balanced against cost and sample size.
- Cognition and function
- Sensitive composites for early change, such as PACC or RBANS, plus CDR-SB for clinical relevance. Digital cognitive tests can add high-frequency resolution.
- In a Phase 1b or small Phase 2, the bar is not to beat standard of care but to show a trajectory that diverges from expected decline.
- Whole-organism aging signals
- DNA methylation clocks as exploratory markers of biological age, such as GrimAge or DunedinPACE, measured in blood. A shift would be hypothesis-generating only. Regulators will not treat these as efficacy endpoints, but they are relevant to the longevity thesis behind the program.
The practical message: pair tight pharmacokinetic and pharmacodynamic evidence of autophagy engagement with standardized Alzheimer’s biomarkers and a pragmatic cognitive battery. That mix can de-risk the mechanism and guide dose and duration for the pivotal work.
How this approach compares to mTOR, senolytics, and GLP-1 drugs
Autophagy is a downstream effect of several longevity interventions, so it is useful to understand how an autophagy-focused drug differs from broader tools. For additional context, see our explainer on rapamycin and mTOR explained.
-
mTOR inhibitors and rapalogs. Rapamycin reduces mTORC1 signaling, which indirectly lifts the brake on autophagy. It has extended lifespan in multiple species and shows promise for aging biology. It also has systemic effects on protein synthesis, immune function, and metabolism, which can be useful or problematic depending on dose and schedule. A selective autophagy enhancer aims to restore intracellular clean-up with less disruption of mTOR’s roles in neurons, including synaptic plasticity.
-
Senolytics. These drugs aim to remove senescent cells that secrete inflammatory factors known as the SASP. Clearing senescent glia may reduce neuroinflammation and improve tissue function, but senolytics address a different failure mode. Autophagy tries to fix cells that are still viable by removing damaged components. Senolytics eliminate cells that are beyond repair. In complex aged tissues, both strategies may be complementary.
-
GLP-1 drugs. GLP-1 agonists reduce neuroinflammation and improve metabolic signaling, and are being tested in Alzheimer’s at scale. They do not primarily target intracellular proteostasis. Combining a proteostasis repair tool with a metabolic anti-inflammatory could be synergistic if safety allows it.
Trial design choices that will matter
-
Stage of disease. Autophagy rescue likely works better when neurons are stressed but salvageable, not when pathology is overwhelming. Early symptomatic Alzheimer’s or prodromal stages are logical. A moderate dementia cohort can establish safety, but efficacy odds will be lower.
-
Duration and dose. Proteostasis repair may need months to translate into biomarker shifts and cognition. A short Phase 1b can show target engagement, but a six to twelve month Phase 2 is the real test. The dose that moves peripheral autophagy markers is not automatically the dose that helps neurons, so titration guided by CSF exposure is key.
-
Background therapies. Many patients will be on anti-amyloid antibodies or symptomatic drugs. Autophagy enhancers could layer on top. Trials should stratify or predefine analyses to clarify add-on effects.
-
Safety watchouts. Overdriving autophagy without matching lysosomal capacity could backfire. Look for gastrointestinal effects, metabolic shifts, or signs of excessive catabolism. In the CNS, watch for worsened inflammation or paradoxical neurodegeneration signals if flux stalls mid-stream. Careful dose escalation and broad safety labs will matter.
What a positive readout would signal
A best-case early signal looks like this:
- Safety and tolerability consistent with a chronic oral CNS drug.
- Measurable CNS exposure.
- Peripheral and CSF pharmacodynamic signs that autophagic flux is up and lysosomal function is engaged.
- Stabilization or improvement in pTau217 and NfL versus expected trajectories over six months.
- A small but statistically reliable benefit on a sensitive cognitive composite, with CDR-SB trending in the right direction.
That pattern would validate autophagy as a human lever for neurodegeneration and aging biology. It would likely pull more capital into proteostasis programs, from small molecules that activate TFEB or modulate cargo receptors, to gene therapies that tune lysosomal biology. It would also embolden developers to test autophagy enhancers in Parkinson’s, Lewy body dementia, and even systemic age-related conditions like sarcopenia or heart failure, where organelle quality control falters with age.
What a negative readout would mean
A negative outcome can be informative in different ways.
-
Clean safety, no target engagement. The drug did not reach the brain or hit its target at tolerable doses. That argues for new chemistry with better CNS penetration or different modalities.
-
Target engagement, no disease biomarker shift. Autophagy tuning alone may be insufficient in established Alzheimer’s. Combination strategies that pair proteostasis repair with anti-amyloid or anti-tau agents could be the next step, or trials must move earlier in the disease.
-
Mixed signals with safety concerns. If raising autophagy creates traffic jams because lysosomes are weak, the field should pivot toward approaches that upgrade lysosomal function first, for example TFEB activation or small molecules that restore lysosomal acidity.
None of these scenarios invalidate autophagy as biology. They refine how and where to apply it, and what companion biomarkers and co-therapies are needed.
The bigger picture for geroscience drug development
The RTR242 trial is a wedge. It tests a general longevity hypothesis in a concrete disease where regulators, clinicians, and families care about outcomes. That wedge matters. Geroscience has made its name in model organisms and prevention talk. To change medicine, it must deliver in controlled human studies with endpoints that matter.
Whatever the readout, two things will advance.
-
A measurement toolkit for cellular clean-up in people. Developers will have to standardize autophagy and lysosome biomarkers that correlate with clinical outcomes. That toolkit will be reused across indications.
-
A trial blueprint for longevity-motivated drugs. The field will learn how to integrate disease-specific endpoints with cross-tissue aging markers, and how to communicate results without overpromising.
If the signal is positive, autophagy could become a pillar of next-generation neurotherapeutics and a beachhead for proteostasis drugs elsewhere. If the signal is negative, it will still sharpen the map. Either way, autophagy’s move into the clinic marks a turning point. A central idea in longevity science is finally being judged where it matters most, in people.