Can Plasma Exchange Reverse Aging or Just the Clock?
A randomized human study in 2025 reports that therapeutic plasma exchange, especially paired with IVIG, lowered multiple epigenetic aging clocks. We unpack mechanisms, durability, safety, costs, and the decisive trial that could prove real longevity benefits.
Key takeaways
- A randomized, single blind, placebo controlled trial in 2025 reported that therapeutic plasma exchange lowered biological age on multiple epigenetic clocks, with the largest shifts when IVIG was added. See the Aging Cell TPE trial abstract.
- Clocks can move quickly when inflammation and circulating proteins change, so younger scores may not equal longer life.
- Safety looked acceptable over the study window, but IVIG adds cost and supply constraints.
- The right confirmatory trial should test durability and hard outcomes like infections, hospitalizations, and function.
The claim: plasma exchange makes you biologically younger
A 2025 human trial found that therapeutic plasma exchange lowered biological age according to several DNA methylation clocks, with the strongest effect in the arm that paired exchange with intravenous immunoglobulin. Participants were healthy adults over 50. The study tested multiple dosing schedules and tracked multi omics shifts across the epigenome, proteome, metabolome, glycome, cytokines, and immune cell composition. In total, 15 epigenetic clocks moved in a younger direction versus placebo, and short term safety looked acceptable. Details and numbers are in the Aging Cell TPE trial abstract.
So did researchers pull a lever on human aging, or nudge biomarkers that are unusually responsive to short term immune and inflammatory changes? We do not know yet. Here is what the clocks measure, what TPE plausibly changes, and how a decisive follow up study should look.
What aging clocks are really capturing
Biological age clocks come in flavors:
- DNA methylation clocks that estimate chronological age or risk weighted age. First generation clocks track age like odometers. Next generation clocks such as hazard based models or pace of aging aim to capture risk and rate of change.
- Multi biomarker composites that blend proteins, metabolites, lipids, inflammatory cytokines, and sometimes clinical labs. These can move quickly with illness, infection, or therapy.
- Single domain clocks like IgG glycan profiles or immune cell composition indices that reflect immune tone and inflammaging.
Each type is useful, yet vulnerable to a common issue. If an intervention suppresses inflammation, shifts circulating immune cells, or dilutes abundant plasma proteins for weeks, several clocks will report a younger state. That may reflect lower near term risk, which is good, but it is not the same as a durable reduction in disease or mortality. The new trial’s broad multi omics canvas is encouraging because it shows coordinated shifts across systems, not just a single score moving. Still, clocks are surrogate endpoints. For longevity, only harder outcomes settle the debate.
How therapeutic plasma exchange might work
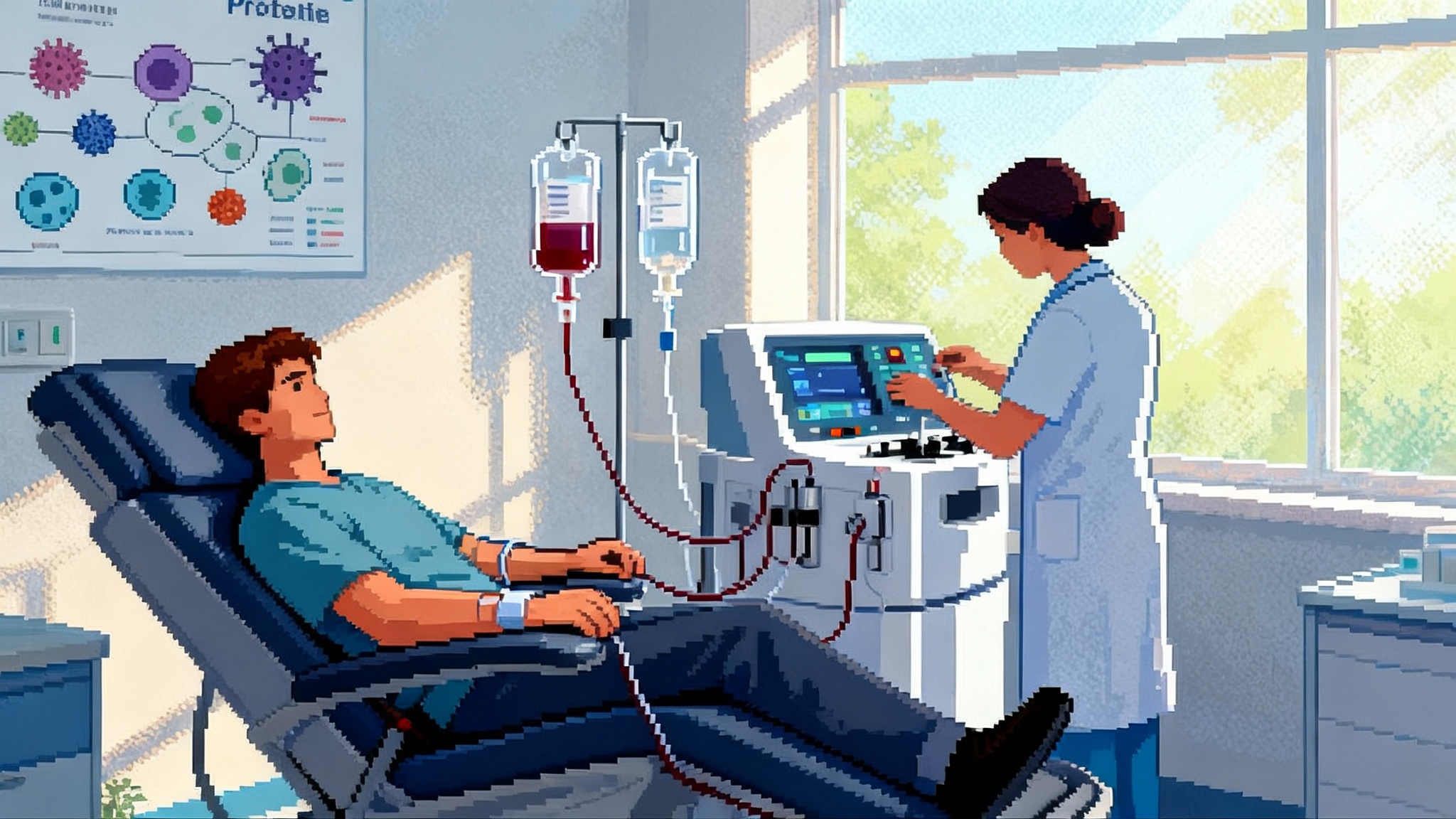
Therapeutic plasma exchange is a decades old hospital procedure. Whole blood is removed from a vein, plasma is separated and discarded, and blood cells are returned with replacement fluid. Replacement is usually albumin in saline, sometimes plasma. A typical session exchanges about one plasma volume and removes large circulating proteins and factors that build up with age and disease.
Three plausible mechanisms explain the biomarker changes:
-
Proteomic dilution and reset. Discarding plasma and replacing it with albumin in saline acutely lowers concentrations of pro inflammatory cytokines, complement components, autoantibodies, extracellular vesicles, and senescence associated secretory phenotype proteins. That dilution can reset signaling toward a less inflammatory milieu. Think of it as replacing murky bathwater, not the pipes.
-
Immune modulation with IVIG. When IVIG is added, Fc receptor saturation, autoantibody neutralization, and dendritic and T cell modulation can push the immune system toward a less reactive state. That could amplify the multi omics rejuvenation signal and explain why the TPE plus IVIG arm outperformed exchange alone.
-
Senescence associated proteins and glycans. Several age linked proteins and IgG glycan patterns tilt toward inflammation with age. Removing and replacing plasma appears to steer those patterns toward a younger distribution. Whether that means fewer senescent cells in tissues is unknown. More likely, the bloodstream reflects changed signaling rather than wholesale cellular rejuvenation.
TPE alone versus TPE plus IVIG
The headline finding is simple. Exchange alone reduced biological age scores. Exchange plus IVIG reduced them more. That raises three practical questions:
- Is IVIG doing most of the work? IVIG is a potent immunomodulator with independent anti inflammatory effects. A clean factorial trial needs an IVIG alone arm.
- How long do effects last? If clocks drift back after weeks, repeated treatments would be needed. Durability separates a transient reset from a trajectory changing therapy.
- Can we predict responders? Individuals with more adverse baseline biomarkers seemed to benefit more. Preselection by inflammatory or metabolic signatures could improve benefit and cost.
How this compares to dilution and young plasma fractions
Animal work showed that diluting old plasma with saline and albumin can rejuvenate tissue repair and cognition in old mice, supporting a dilution reset model. Separate groups report that concentrated fractions from young or cord plasma can shift epigenetic clocks and clinical biomarkers in small human studies. The common thread is that changing the circulating milieu can move markers of aging. The open questions are durability, magnitude in humans, and translation to disease endpoints. For a broader view of translating aging biology through disease endpoints, see our look at Retro’s autophagy pill enters human trials.
Biomarker signal versus clinical benefit
Many methylation and proteomic clocks predict mortality, cardiovascular events, and frailty. That is why clock drops are exciting. The problem is translation. We still lack randomized human evidence that lowering several clocks reduces disease, hospitalizations, or death. The closest analogs are lifestyle and pharmacologic studies where clock shifts align with improved cardiometabolic risk factors, but long term outcomes remain sparse. For context on how risk factor changes can scale to population outcomes, see GLP 1s and the new longevity math.
What a decisive confirmatory trial should look like
If you were designing the next study to convince payers, regulators, and skeptics, here is a pragmatic plan:
- Population: adults 55 to 80 with elevated inflammatory or metabolic markers, not acutely ill. Stratify by baseline inflammatory score and frailty index to study responders.
- Arms: TPE with albumin, TPE plus IVIG, IVIG alone, and sham apheresis. If feasible, include a neutral blood exchange arm to test dilution explicitly. Keep replacement fluid consistent across arms.
- Blinding: double blind participants and outcome assessors. Consider placebo IVIG to maintain blinding.
- Dosing: prespecify a loading phase, for example three exchanges in six weeks, then maintenance every one to three months. Test two schedules to map durability.
- Primary endpoints at 6 and 12 months: composite of serious infections, days hospitalized, and change in frailty score.
- Secondary endpoints: vaccine response titers, cardiometabolic markers, physical performance, cognitive tests, and quality of life.
- Exploratory endpoints: a limited, prespecified panel of epigenetic clocks, proteomics, glycomics, and immune repertoire with multiplicity control.
- Safety: monitor hypotension, citrate induced hypocalcemia, bleeding risk with low fibrinogen, allergic reactions to replacement fluids, line infections, and IVIG related complications such as headache, thrombosis, aseptic meningitis, and renal injury. Independent monitoring is essential.
- Durability and rebound: follow participants to 18 to 24 months to see if biomarkers rebound and whether clinical gains persist.
- Health economics: capture costs per quality adjusted life year and model budget impact. Without reimbursement, uptake will be limited.
Safety realities, not hypotheticals
Therapeutic plasma exchange is widely used for specific diseases and is generally safe in experienced hands. Common issues include transient hypotension, hypocalcemia from citrate, mild allergic reactions, and venous access problems. Serious events such as anaphylaxis, major bleeding, or line infections are uncommon but real. Risk rises when fresh frozen plasma is used as replacement fluid rather than albumin. IVIG adds frequent headaches and rare but serious thrombosis or kidney injury, especially in older or high risk patients. A longevity program would need strict protocols for calcium supplementation, fibrinogen monitoring, and careful selection for vascular access and clotting risk. For coverage context, see the ASFA 2023 therapeutic apheresis guideline.
Cost, access, and the regulatory and payer maze
TPE devices and disposables are cleared for many indications. IVIG is a licensed biologic with labeled uses, and physicians can prescribe it off label. What programs cannot do is market TPE plus IVIG with longevity claims without evidence. Payers cover TPE for specific conditions graded by professional guidelines, not for elective movement of aging biomarkers, which means self pay unless trials show durable clinical benefit. For how novel longevity claims may find a regulatory path, see our coverage of the dog longevity blueprint for humans.
Who should consider this now
- Healthy adults: evidence is not mature enough to justify routine TPE for longevity.
- Patients with accepted indications: new data may be a welcome bonus rather than the reason to treat.
- Researchers and early adopters: trials are the right venue to answer questions about durability, safety, and outcomes.
A pragmatic bottom line
The 2025 human study is a credible signal that large scale changes to the circulating proteome and immune environment can make multiple aging clocks read younger, at least for a time. That is not the end of the story. It is the beginning of faster experiments aimed at outcomes people care about. If the next generation of trials shows fewer infections, shorter hospital stays, better vaccine responses, and improved function, the clocks will have earned their keep as decision tools. If not, we will have learned something equally valuable about the limits of biomarker rejuvenation.
Until then, treat TPE and TPE plus IVIG as promising tools in search of the right use case. Keep enthusiasm high, keep claims modest, and push for rigorous trials focused on durability, safety, and hard endpoints.