Partial reprogramming nears trials as the eye goes first
A wave of 2025 OSK data in primates and metabolic models is pulling partial epigenetic reprogramming toward the clinic. Learn why ophthalmology leads, how control systems make it feasible, and what to watch next.
The breakthrough moment for reprogramming
Partial epigenetic reprogramming has moved from a provocative mouse trick to a translational race. Over the past year, the strongest signals have come from the eye. Life Biosciences reported nonhuman primate results in a model of optic nerve stroke showing improved pattern electroretinograms and axon survival after intravitreal delivery of an inducible OSK gene therapy. The program, ER-100, uses Oct4, Sox2, and Klf4 without c-Myc to avoid uncontrolled proliferation, and its dosing is switched on with doxycycline. These primate data are company reported but detailed and consistent with earlier rodent work, which is why they have become the lead indicator for first-in-human testing Life Biosciences primate data at AAO.
Outside the eye, the field has seen the most concrete systemic signal to date in mice. Rejuvenate Bio published a peer-reviewed study showing that systemic adeno-associated virus delivery of inducible OSK in very old wild-type mice increased remaining lifespan by about 109 percent and improved frailty scores. That does not guarantee human benefit, but it validates that carefully controlled OSK can shift whole-body physiology in mammals lifespan extension with inducible OSK.
Together, these two pillars explain both the excitement and the caution. The eye offers a way to localize expression and measure function with unusual precision. The mouse lifespan data justify continued investment, while underscoring that control is the core technology, not a footnote.
Why the eye is the beachhead
The eye is where partial reprogramming is most likely to enter humans first. Four practical reasons make it a natural starting point.
-
Local delivery with a small dose. Intravitreal injections place the therapy close to retinal ganglion cells with microliter volumes. That keeps systemic exposure low and simplifies dose ranging. It also means manufacturing demands are more modest than for body-wide delivery.
-
Immune privilege and a known playbook. The eye has a degree of immune tolerance. Ophthalmology has two decades of experience with vectorized therapies. Routine anti-VEGF injections have normalized the clinic workflow, and approved ocular gene therapies have set precedents for vector choice, dosing, and safety monitoring. See our analysis of UBX1325’s 2025 eye readouts for how ophthalmology measures fast, objective endpoints.
-
One eye as a built-in control. In early dose-escalation studies, the untreated eye can serve as an internal comparator for safety and sometimes for function, reducing variance and sample sizes.
-
Clean functional endpoints. Pattern electroretinography, visual fields, optical coherence tomography, and ganglion cell complex thickness provide objective readouts within months. For regulators and payers, a clear signal with a clear clock is persuasive.
The first indications are likely non-arteritic anterior ischemic optic neuropathy and primary open-angle glaucoma. In both, retinal ganglion cells die and their axons degenerate. Conventional drugs cannot replace lost axons. Reprogramming aims to push damaged cells into a regenerative state without erasing their identity. That is a precise job for a localized therapy with a switch.
Control systems are the product
The difference between regeneration and risk is control. Three design choices matter most.
- Factor selection. OSK excludes c-Myc to reduce oncogenic pressure. That helps keep cells from overshooting into pluripotency. Some groups are adding repressor elements that bias OSK toward a youthful somatic program, not an embryonic one, by tuning relative expression or by pairing OSK with lineage-specific transcriptional cues.
- Induction and dose control. The most common clinical-grade switch today is the tetracycline system. A reverse tetracycline transactivator drives a promoter that turns on only in the presence of doxycycline. In the primate eye studies, investigators combined an intravitreal vector with daily oral doxycycline. Clinically, that turns the genetic therapy into a drug-controllable device. Turn the dial up or down. Pause during intercurrent illness. Stop if biomarkers drift.
- An emerging toolbox beyond tetracycline. Degron-based switches place a small tag on the OSK proteins that makes them unstable unless a benign small molecule is present. Add drug, the factors stabilize. Remove drug, they are degraded. MicroRNA target cassettes can silence OSK in off-target cell types, for example by making transcripts susceptible to microRNAs that are enriched in glia or photoreceptors but low in retinal ganglion cells. These layers can be stacked. Think of reprogramming as a mixing board, not an on off lamp.
Temporal patterning also matters. Duration and spacing are as important as peak levels. In mice, intermittent cycles often outperform continuous expression. In the eye, early readouts like pattern electroretinogram can be used to titrate cycle length. A practical rule is to start low and slow, with pre-specified gates to escalate only after objective signals and clean safety labs.
The delivery that makes it feasible
Vector choice sets the safety envelope and the reach.
-
Adeno-associated virus for ocular delivery. AAV2 and related capsids have long track records in the inner retina after intravitreal injection. They are efficient at transducing retinal ganglion cells, particularly when paired with promoters like synapsin or thy1 that enrich expression in neurons. Dual-vector designs that split the switch and the payload allow tighter control but require both vectors to co-transduce the same cells. Single-vector designs simplify logistics but can complicate promoter arithmetic. In the clinic, the default path is likely an AAV2-class capsid, a ganglion cell–biased promoter, and a tetracycline switch, with microRNA off-switches to reduce leakage in non-target cells.
-
Lipid nanoparticles and messenger RNA for pulsed exposure. For the eye, a lipid nanoparticle carrying messenger RNA could deliver short pulses of OSK to test whether functional gains can be induced without any persistent transgene. Pulsed messenger RNA lowers the risk of long-tail expression but may require frequent dosing, which is less attractive for intravitreal delivery. It may find its place in corneal indications or periocular injections where repeated dosing is easier.
-
Capsid engineering for systemic disease. For metabolic or hepatic indications, the capsid and promoter choices change. AAV8 or AAV9 reach liver well. In mice, systemic AAV9 carrying inducible OSK can shift frailty and lifespan. Translating that to humans will require lower-immunogenicity capsids, improved manufacturing yields, and new safety valves to shut down expression in the rare cell that loses fate control. Those are solvable with current technology, but they set the bar for systemic indications higher than for the eye.
How recent metabolic disease data fits
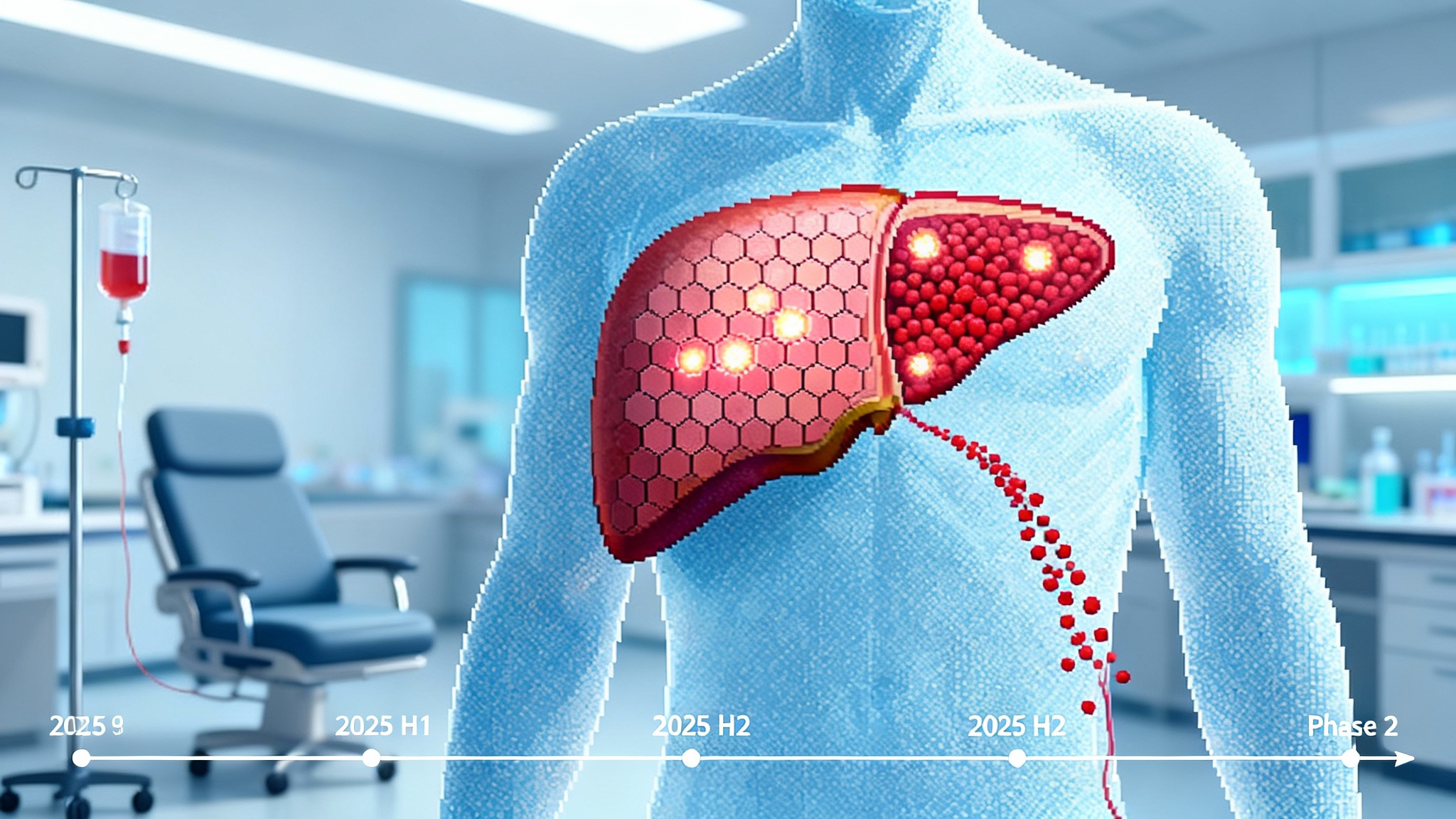
Metabolic disease models are becoming the second testing ground for partial reprogramming. Several groups have reported that OSK shifts methylation states and gene expression programs in the liver and adipose tissue in aged mice. Companies are positioning liver-focused candidates for metabolic dysfunction–associated steatohepatitis, with internal data packages that track histology, mitochondrial function, and senescence markers. The goal is not to make hepatocytes embryonic. It is to push them back toward a more youthful metabolic set point where lipids are handled more cleanly and inflammatory circuits are calmer.
This is a harder path than the eye for two reasons. First, the required dose is larger, so vector-related toxicities and pre-existing neutralizing antibodies matter more. Second, functional endpoints arrive slowly and are confounded by behavior and diet. That is why metabolic disease programs are prioritizing biomarkers that move in weeks, like noninvasive imaging of liver fat and blood-based fibrosis panels, while they build toward histology. For measurement strategy, see our trial-grade biomarker playbook.
Safety is the hinge
The biggest risk is dedifferentiation. If cells overexpress OSK for too long or at too high a level, they may lose identity and proliferate. The defense is a multilayered set of safety features.
- Conservative factor choice. Excluding c-Myc lowers transformation risk.
- Tunable expression. Tetracycline switches or degrons allow dose reduction and fast shutoff.
- Cell biasing. Promoters and microRNA cassettes limit expression to the target cell type.
- Treatment windows. Cyclic dosing avoids continuous pressure.
- Kill switches. Preclinical constructs can include drug-gated suicide genes that eliminate cells if they cross a risk boundary. These are not likely in first ocular trials, but they will be important for systemic programs.
In the eye, the clinic can also monitor for early warning signs with optical coherence tomography and visual fields. In the liver, safety monitoring will depend on sensitive chemistries, imaging, and circulating tumor DNA assays designed to catch off-target clones. These are not afterthoughts. They are part of the product.
Why 2025 feels different
Three things are new.
- Nonhuman primate signals in the eye with a clinical-ready control system. That closes the translation gap that stymied earlier work in rodents. The anatomy and dosing of the primate eye are close to humans. The measured endpoints map directly to what trials will collect.
- A credible systemic signal in very old mice. The lifespan and frailty changes indicate OSK can move the needle on multi-organ function when controlled well. That makes metabolic and hepatic programs more than a moonshot.
- A maturing engineering toolkit. The field is now fluent in switches, tissue targeting, and manufacturing. It looks more like gene therapy with a new kind of cargo than a one-off biology experiment.
What to watch in the next 12 to 24 months
Milestones that will decide whether partial reprogramming becomes a flagship longevity modality are specific and testable.
-
First-in-human dosing in an optic neuropathy. A single ascending dose study will answer whether inducible OSK is tolerated with intravitreal AAV and systemic doxycycline. Expect a cautious design that treats one eye, starts with low vector genomes per eye, and uses a conservative induction schedule. The earliest signals will be safety and pharmacodynamics, for example vector shedding, local inflammation, and evidence that the switch works when doxycycline is started and stopped.
-
Early functional readouts. Within months, look for changes in pattern electroretinogram and structural markers like retinal nerve fiber layer thickness. A small but consistent difference versus the untreated eye would be meaningful. Visual fields and patient-reported outcomes will take longer and be noisier, but they are the bridge to registrational endpoints.
-
Manufacturing and dose. Vector yields and quality control are a gating factor for any gene therapy. Successful release of multiple good manufacturing practice lots at the clinical dose will be a strong green flag. Failures here often delay programs more than biology does.
-
New control layers in the clinic. Watch for second-generation ocular candidates that replace the tetracycline switch with a nonantibiotic small-molecule stabilizer or add a microRNA cassette to reduce leakage. These are incremental but important steps toward systemic safety.
-
Metabolic disease packages that are hard to fake. In the liver, look for paired animal data that combine histology, single-cell transcriptomics, and methylation clocks with functional metrics like fat fraction on imaging. Groups that can reproduce the signal across two species and two diets will separate from the pack.
-
The first large-animal systemic study with a shutoff that works under stress. Systemic programs will need to show that the switch can be triggered to shut down in a febrile or inflammatory setting, not just in steady state. That demonstration would build confidence for first-in-human systemic trials.
-
Regulatory clarity on class-wide risks. Expect guidance on long-term monitoring for insertional events, even though adeno-associated virus rarely integrates. The field should also define standard panels for circulating tumor DNA and methylation-based surveillance. Shared standards reduce surprises and speed consensus.
Practical implications for teams and investors
-
For companies. If you are an ocular program, invest in patient-friendly doxycycline protocols and home-based adherence tools. Doxycycline is safe but can disturb the microbiome. Prophylaxis and monitoring plans will make or break adherence. Build a visual function core lab early so that small signals are measured well.
-
For systemic aspirants. Prioritize capsid and promoter engineering as much as factor biology. If your capsid reduces neutralizing antibody exclusion by 20 to 30 percent, you gain a practical advantage in eligibility and redosing. Programs tackling inflammaging should track adjacent levers like necroptosis blockers entering trials.
-
For the ecosystem. Fund shared toxicology and biodistribution datasets for common switches and promoters. A public baseline lowers duplication and speeds iteration.
-
For clinicians. Prepare patients for a device-like experience. Reprogramming is a genetic implant that you modulate with a pill. That needs clear counseling, adherence plans, and contingency instructions.
A grounded forecast
If the first ocular studies show clean safety and a consistent electrophysiology signal within the first cohorts, the sector will move quickly. A positive result would validate the core premise that cells can be coaxed back toward a youthful state without losing their identity. It would also de-risk control systems and ocular delivery, opening the path to other inner retina diseases.
If the ocular signal is flat or safety is noisy, the field will regroup around better switches and promoters. That would not end reprogramming, but it would push systemic ambitions further out while the engineering catches up.
Either way, the next two years will trade poetry for proof. The eye is giving reprogramming its first fair test, with a switch, a small dose, and a clock that ticks fast enough to matter. That is how a breakthrough becomes a therapy.