Pig kidneys in humans: the new longevity frontier
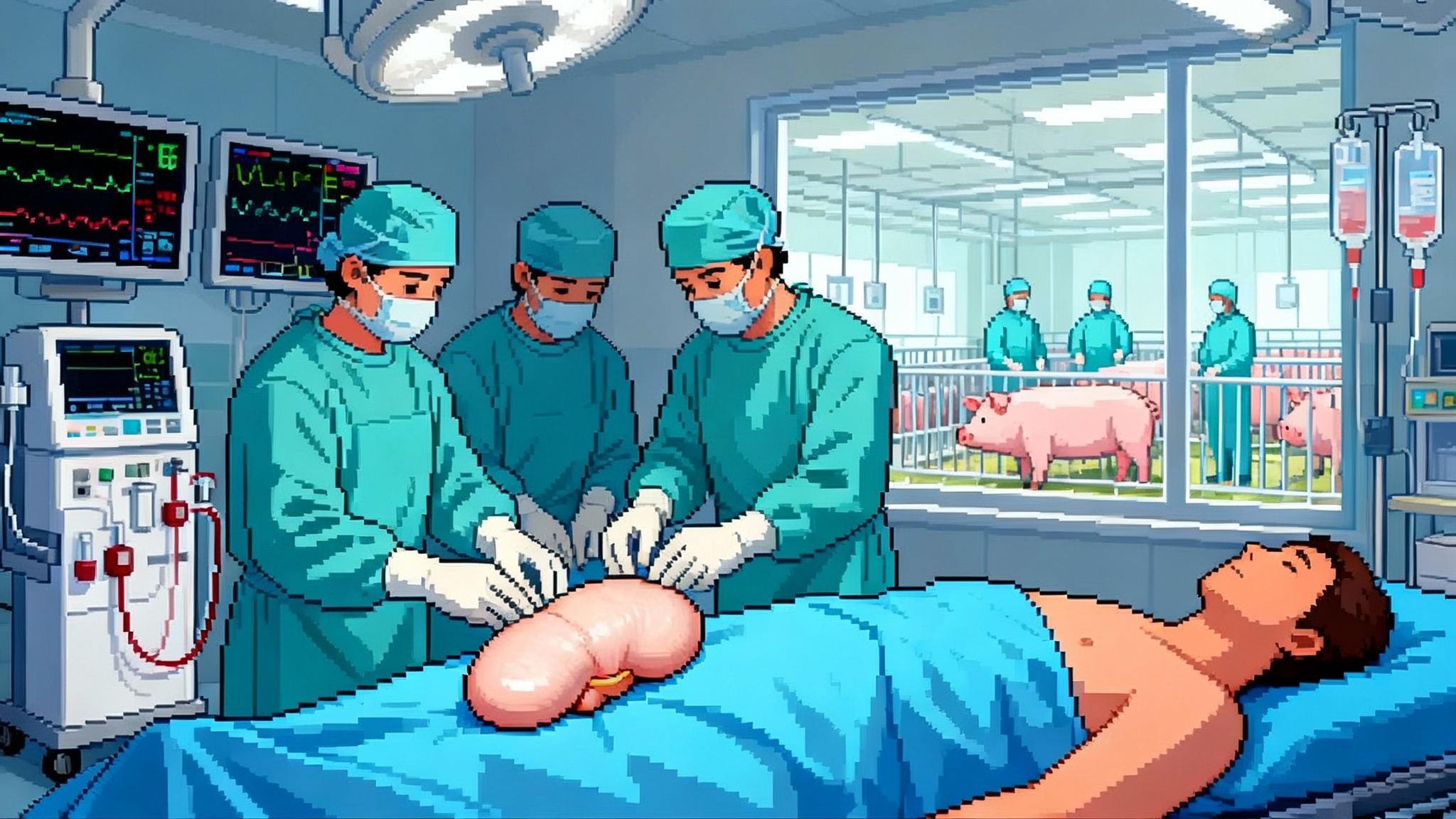
With the FDA clearing the first clinical trials of pig-to-human kidneys and a second living-recipient transplant at Mass General, xenotransplantation just moved from theory to clinic. Here is how it could change lifespan and healthspan, and what to watch next.
The week pig kidneys left the lab
Xenotransplantation stopped being a thought experiment the moment the Food and Drug Administration FDA cleared kidney xenotransplant trials in early February 2025. Within days, Massachusetts General Hospital confirmed its second living recipient had received a gene edited pig kidney on January 25, 2025, and left the hospital off dialysis a week later. Add a separate FDA clearance in April for a study that uses pig livers as an external support for failing human livers, and a once speculative field now has multiple clinical entry points. The signal to longevity watchers is clear. Supply constrained organs might soon be a manufactured product, not an accident of altruism.
This is a moment to be excited and sober at the same time. Kidneys are the logical first target. They are the most demanded organ, the technology stack for dialysis offers a safety net if a graft fails, and the clinical endpoints are measurable on practical time horizons. Yet the calculus for human lifespan and healthspan depends on more than surgical success. It depends on whether engineered pigs, immunosuppression regimens, surveillance systems, and regulators can jointly turn a handful of compassionate use cases into a reliable therapy.
Why kidneys change the mortality curve first
Dialysis keeps people alive, but it is not a longevity plan. Annual mortality on dialysis remains high and quality of life is impaired by time on machine, cardiovascular stress, and infection risk. Transplant, not dialysis, is the proven survival and healthspan intervention. The problem is supply. With tens of thousands waiting for a kidney at any moment and far fewer organs available, patients spend years accumulating comorbidities that reduce the benefit even when a kidney arrives.
A scalable xenokidney shifts that math in three ways:
-
Shorter time to transplant. If wait times compress from years to months, mortality on the list drops and recipients arrive to surgery in better shape. That alone increases post transplant survival and reduces cardiovascular events.
-
More transplants for sensitized patients. Alloimmunized patients are hard to match to human donors. A gene edited pig kidney can be manufactured without the antigens that drive hyperacute rejection and may be paired with a tailored immunosuppressive plan. That unlocks transplants for people who would otherwise never get one.
-
Fewer discarded organs. Human kidneys are sometimes declined because ischemic time or donor factors make outcomes uncertain. A manufactured organ arrives on schedule, fresh, and standardized. Predictability reduces waste and planning friction across the transplant center.
Translate that into years of life. Even if early xenokidneys deliver survival similar to marginal deceased donor kidneys, the immediacy of access can improve both life expectancy and healthy time lived off dialysis. The first large gains accrue not to the ultra healthy but to the people stuck on dialysis who are good surgical candidates yet blocked by a queue that outlives them.
What success looks like in 2025 and 2026
Kidney trials in 2025 are designed to answer a narrow question. Can a gene edited pig kidney keep a person alive and off dialysis for months without unacceptable safety signals. The bar is not five year graft survival. The bar is proving clinical viability on a short timeline and safety under intensive surveillance.
Near term success criteria to watch:
- Ninety day graft survival. This is the first non negotiable. If most early recipients are alive and dialysis independent at ninety days, the field advances. If not, programs will reset.
- Renal function trajectory. Estimated GFR above 30 ml per minute at ninety days, trending up or stable, with minimal proteinuria. Flat or collapsing eGFR curves are trouble even if dialysis is avoided.
- Hospital length of stay. Fast recovery suggests the procedure can scale. Prolonged ICU time and readmissions make the therapy brittle and expensive.
- Adverse events. Serious infections, thrombotic complications, and uncontrolled rejection episodes will determine dose and choice of immunosuppression in the next cohorts.
- Patient reported outcomes. Energy, diet, freedom from dialysis logistics. Healthspan is more than lab values.
If those boxes are mostly checked, expect cohort expansion before the end of 2025 and additional centers seeking authorization in 2026. If the field hits turbulence at ninety days, teams will iterate on edits and drug regimens rather than retreat, but timelines will stretch.
From kidneys to hearts and livers
Kidneys are the beachhead, not the end game. Heart xenotransplantation already has individual human cases that survived weeks to months. The engineering targets are similar, yet hearts lack a dialysis like backup and are more vulnerable to rejection and infection. The payoff is enormous. Advanced heart failure kills quickly and unpredictably. If xenografts can provide bridge to transplant or destination therapy for even a subset of patients, the mortality benefit is immediate. Expect hearts to trail kidneys by several years because the safety margin is thinner and the clinical windows for testing are shorter.
Livers may advance on two tracks. One is full organ replacement, which shares many of the immune risks of hearts. The other is using a pig liver outside the body as a temporary filter for patients with acute liver failure. The FDA cleared extracorporeal pig liver trial in April 2025, and it is conceptually attractive because it buys time. Some human livers recover if you can bridge a patient through the inflammatory storm. An external pig liver gives the native organ rest without the irreversible step of transplantation. If that works, it reduces ICU mortality and may reduce the number of people who ever need a liver transplant.
The safety ledger that will make or break adoption
Every xenotransplant balances immune risk, infectious risk, and drug toxicity. The current playbook attacks immune compatibility and zoonoses in parallel.
- Genetic edits. Pigs used for kidneys now carry multiple edits to remove carbohydrate antigens that trigger hyperacute rejection, and they add human complement regulatory genes to reduce vascular injury. Some lines also inactivate porcine endogenous retroviruses that could theoretically infect human cells. Fewer incompatible targets and more human friendly surfaces reduce the front end immune storm.
- Immunosuppression. Expect regimens that go beyond standard kidney transplants. Costimulation blockade drugs, complement inhibitors, and possibly targeted therapies that blunt the specific anti pig response are likely. The trade off is infection and malignancy risk, so the field will test minimal effective dosing in tight step ups.
- Infection surveillance. Recipients will undergo frequent PCR based viral panels, metagenomic sequencing in some centers, and lifelong follow up as a condition of participation. The goal is early detection of zoonotic events and the ability to trace and contain them.
For the public, the litmus test is not zero risk. It is whether risks are lower than the status quo and managed transparently. Dialysis already carries infection risk. Human to human transplants already transmit pathogens on occasion. Xenotransplantation has to clear a higher bar because the source is a different species, but the tools to monitor and respond have never been better.
Manufacturing organs, not just harvesting them
The unglamorous center of this story is animal husbandry. To deliver thousands of safe organs, companies must operate biosecure, designated pathogen free herds that meet medical manufacturing standards.
What that entails:
- Barrier facilities. Positive pressure barns, HEPA filtration, strict personnel flows, shower in and out. Caesarean derivation and isolated rearing to eliminate vertical transmission of pathogens.
- Clean feed and water chains. Everything that enters the herd is validated. Supply disruptions can compromise biosafety.
- Continuous health surveillance. Sentinel animals, routine PCR panels, and environmental sampling. Genomic surveillance of pig lines to confirm edits, check for off target changes, and verify PERV status across generations.
- Organ logistics. Pickup, preservation, and delivery tuned to surgical schedules. Normothermic perfusion devices that keep an organ physiologic from barn to table help maintain quality and reduce ischemic time.
Scaling from dozens to thousands of organs per year will stress each link. The advantage over human donation is controllability. If a variant emerges in the herd, you can cull a line, sanitize the facility, and restart from frozen embryos. Organ production becomes a reproducible industrial process, not a scramble driven by tragedy and timing. Similar standardization pressures are reshaping cardiometabolic prevention, as seen in the one and done model of PCSK9 one and done editing.
The regulatory path and realistic timelines
Regulators have been pragmatic. The first kidney trials are small, staged, and focused on safety, with independent monitoring and predefined expansion rules. The design looks like this. Treat a first cohort of carefully selected end stage renal disease patients under an investigational new drug. Follow them closely for twelve to twenty four weeks. If predefined safety and performance criteria are met, expand to a larger group. Meanwhile, require lifelong follow up and data sharing into a centralized registry. For a preview of how novel longevity interventions can move from animals to people, see our take on the FDA dog longevity blueprint.
If the first six to ten patients do well through mid 2025, more centers will join and total enrollment will grow into the dozens. A pivotal study that supports a biologics license is unlikely before 2027 at the earliest, and broader availability before the end of the decade is still an optimistic scenario. That said, compassionate use and expanded access pathways can help specific patients sooner if centers build experience and safety holds.
For the liver support device, the bar is different. Benefit can be measured in days saved, progression to transplant avoided, or organ recovery achieved. If early cases show a clear reduction in ICU mortality without new safety problems, that program could move faster because the intervention is temporary and removable.
The watchlist for the first cohorts
A practical checklist for readers tracking the field over the next eighteen months:
- Ninety day graft survival and dialysis independence.
- eGFR levels at thirty, sixty, and ninety days, with proteinuria trends.
- Rejection episodes confirmed by biopsy, especially antibody mediated events.
- Anti pig antibody titers and crossmatch results over time, which indicate whether the immune system is adapting or escalating.
- Opportunistic infections, particularly viral reactivations and any unexpected swine related signals.
- Hospital days, readmissions, and procedure related complications.
- Drug regimens, dose intensity, and adverse effects as teams dial down to the minimal effective immunosuppression.
- Quality of life scores and return to work or normal activity rates.
The longevity calculus, by the numbers
Consider two scenarios. In the first, xenokidneys achieve a ninety day success rate above 80 percent with acceptable safety, and one year graft survival that approaches the lower end of deceased donor outcomes. Centers roll in more patients with careful selection. Within two years, a few thousand people avoid years of dialysis and its cumulative cardiovascular damage. That translates directly to lives extended and healthier years lived. Population level gains can compound alongside other interventions that move mortality curves, such as the GLP-1 mortality forecast.
In the second scenario, early safety issues force tighter selection and slower growth. The path still moves forward, but the near term population level impact is modest. The key difference is not the science alone, it is whether manufacturing and surveillance keep the risk predictable and low enough that regulators and payers can say yes for more than a handful of cases.
Even in the conservative case, one special population benefits quickly. Highly sensitized patients who are poor candidates for human kidneys but can be matched to a gene edited pig kidney get a viable option. For them, xenotransplantation is not a theoretical promise, it is a path off a machine that was never a path to longevity.
What could slow things down
- Unexpected infections. A detection of a novel pig to human transmission would trigger immediate investigations and possibly pauses. The response will determine whether confidence returns quickly.
- Allo to xeno immune surprises. If the human immune system develops new classes of antibodies that blunt current edits, companies will need a new editing cycle. That could add years.
- Drug toxicity. If costimulation blockade or complement inhibition at effective doses causes too many infections or malignancies, regimens will need to be redesigned.
- Public perception and ethics. Biosecurity missteps or animal welfare scandals could harden opposition. Transparent, audited husbandry programs help here, as do clear consent processes and long term patient support.
The accelerationist case for measured speed
There is a temptation to sprint. The organ shortage is a daily tragedy and the tools finally exist. The smarter approach is controlled acceleration. Small cohorts, frequent looks, rapid iteration on edits and drugs, and data sharing across centers. The aim is to reach a stable operating point where a xenokidney is predictable enough to roll out to many patients and a xenoliver support device can be placed in ICUs that need it.
Engineering and regulation can be friends in this case. A standardized organ should make trial design cleaner, endpoints sharper, and post market surveillance more informative. If companies treat pigs like medical manufacturing rather than agriculture, the system will learn faster and fail safer.
Bottom line
Kidneys are the tip of a spear aimed at one of the most stubborn bottlenecks in medicine. With the first trials underway, the question is not whether xenotransplantation can work, but whether it can scale safely and predictably. If the early ninety day data are solid, the near term impact will be fewer deaths on dialysis, more people living free of machines, and cleaner paths to transplant for those who had none. Hearts and full liver replacements will follow more slowly, while external liver support could save lives sooner by buying time.