One-and-done LDL therapy: VERVE-102’s 2025 fast track
In April 2025, the FDA put VERVE-102 on Fast Track and early Heart-2 results showed deep LDL reductions after a single infusion. Here is what PCSK9 base editing could mean for prevention, safety, durability, and the road to Phase 2.
Breaking news for cholesterol prevention
In April 2025, a quiet but defining moment for heart health arrived. The Food and Drug Administration granted Fast Track designation to VERVE-102, an in vivo base editing medicine that aims to permanently switch off PCSK9 in the liver after a single intravenous infusion. Verve disclosed the designation on April 11, 2025, and outlined a plan to accelerate development and review for high-risk patients who need deeper low-density lipoprotein lowering. See Verve’s release on the FDA Fast Track for VERVE-102.
Three days later, on April 14, 2025, the company shared the first-in-human results from the Heart-2 Phase 1b study. In 14 participants across the first three dose cohorts with at least 28 days of follow-up, a single infusion produced dose-dependent reductions in both circulating PCSK9 and low-density lipoprotein cholesterol. At the 0.6 mg/kg dose, the mean low-density lipoprotein reduction was 53 percent and the maximum reduction reached 69 percent in one participant. The initial analysis reported no treatment-related serious adverse events and no clinically significant laboratory abnormalities through the early follow-up window. Those data are summarized in Verve’s initial Heart-2 clinical results.
Why a single edit to PCSK9 matters
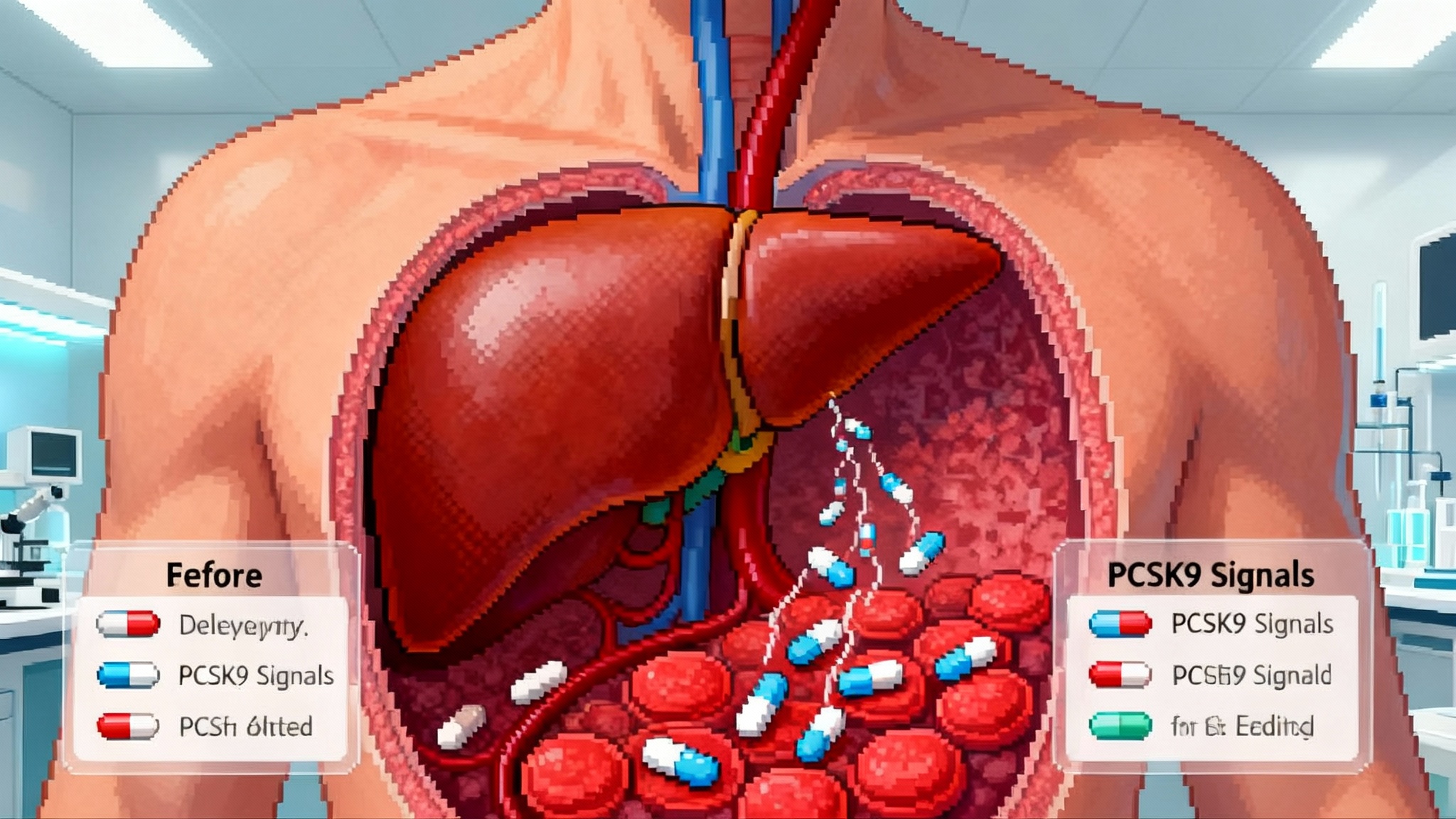
PCSK9 is a traffic cop for the low-density lipoprotein receptor on liver cells. When the gene is active, more PCSK9 protein circulates and marks the receptor for recycling, leaving fewer receptors to clear cholesterol from the blood. People born with loss-of-function variants in PCSK9 tend to have lifelong low low-density lipoprotein levels and very low rates of atherosclerotic events. Drugs that inhibit PCSK9 have already proven the principle. Monoclonal antibodies like evolocumab and alirocumab lower low-density lipoprotein by roughly 60 percent when added to statins. A small interfering RNA therapy, inclisiran, typically lowers it around 50 percent with twice-yearly maintenance dosing.
The difference now is the delivery and the timeline. Instead of blocking PCSK9 again and again, VERVE-102 attempts to edit the DNA inside liver cells so that PCSK9 is turned off for the long term. If it works as designed, it could shift prevention away from decades of adherence to an install-and-forget intervention.
Think of your liver as a massive factory with millions of identical machines. Most medicines pause a troublesome machine only while the drug is around. Base editing is more like swapping a faulty instruction card inside the machine. Once the card is changed, the machine stops stamping out PCSK9 for good, and other useful processes keep running.
How base editing actually works
Traditional CRISPR nucleases cut both strands of DNA and rely on cellular repair to paste things back together. That can be powerful but also unpredictable. Base editing does not make a double-strand break. Instead, it uses a programmable enzyme to change a single letter of DNA, for example changing A to G. VERVE-102 packages two pieces of RNA inside a lipid nanoparticle to reach the liver. One piece is messenger RNA that instructs the cell to produce the adenine base editor. The second is a guide RNA that takes the editor to the PCSK9 site. The result is a precise letter change that stops the PCSK9 gene from working in that cell.
VERVE-102 also uses a liver-targeting ligand called GalNAc on its lipid nanoparticle shell. That feature provides two ways into hepatocytes through the low-density lipoprotein receptor and the asialoglycoprotein receptor. The aim is to improve delivery, reach more liver cells with less dose, and temper the safety worries raised by an earlier formulation used in VERVE-101.
What the Heart-2 data tell us so far
Early results do not decide the story, but they offer a signal. Heart-2 enrolled adults with heterozygous familial hypercholesterolemia and others with premature coronary artery disease who needed additional low-density lipoprotein lowering despite standard therapy. The first three cohorts received 0.3, 0.45, and 0.6 mg/kg as a single infusion. The higher the dose, the deeper the average low-density lipoprotein drop. The time-averaged reductions from day 28 onward suggest the effect persists beyond the immediate post-infusion window, which is what you would expect if the edit is in place and edited cells are now producing less PCSK9.
The safety snapshot matters. After a pause in 2024 to investigate thrombocytopenia and liver enzyme elevations in a patient who received the earlier VERVE-101 formulation, Verve redesigned delivery for VERVE-102. In Heart-2’s initial analysis, investigators reported no treatment-related serious adverse events, no clinically significant lab abnormalities, and only one moderate infusion reaction that resolved with symptomatic care. The follow-up remains short, so clinicians and regulators will focus on longer-term safety, especially as dose escalation continues and the planned fourth cohort at 0.7 mg/kg completes.
Durability, dosing, and the possibility of true one and done
If the edit is successful, it should last as long as the edited hepatocytes remain in the liver. Hepatocytes do turn over slowly, but not so fast that the effect vanishes within months. The open question is how much editing is needed to keep low-density lipoprotein suppressed for years, and whether a single edit session can achieve that fraction safely. Heart-2 is also analyzing pharmacodynamics by total RNA dose in milligrams, which may become a more useful yardstick than weight-based dosing for lipid nanoparticle gene editors. If total RNA rather than mg/kg proves predictive, future dosing could be simplified and standardized.
A practical wrinkle is redosing. Many gene editing approaches face immune responses to the editor protein, which can make repeat administration difficult. That is another reason the threshold for success is high. One visit needs to produce enough editing to deliver long-lasting benefit without the option to simply top up later.
Safety checkpoints and off-target risks
Two broad classes of risk need watching. The first is acute toxicity from the delivery system and the editing payload. Lipid nanoparticles can trigger infusion reactions and transient liver stress. That is why trials monitor platelets and liver enzymes closely in the first days and weeks. Heart-2’s initial readout is reassuring but cannot rule out rare events or late signals.
The second is off-target editing. Base editors can cause unintended letter changes at genomic sites that look similar to PCSK9 or in the RNA of the cell. Companies address this with careful guide selection, exhaustive off-target prediction, and deep sequencing of candidate sites in liver tissue and blood cells. Regulators will also ask for longer-term monitoring for cancer signals. The risk is probably lower than approaches that make double-strand breaks, but it is not zero. Patients and physicians should expect a careful conversation about unknowns and the follow-up commitments that come with a gene editing therapy.
The road to Phase 2 and how commercialization could unfold
Verve has said it expects final data from Heart-2’s dose escalation in the second half of 2025 and plans to initiate a Phase 2 trial in that same window, pending regulatory clearance. The program now benefits from Fast Track, which enables more frequent engagement with the agency and rolling review if and when an application is filed.
How might approval work for a one-time editor that lowers low-density lipoprotein? Regulators have accepted low-density lipoprotein reduction as a surrogate for clinical benefit in high-risk populations, with hard outcome trials following post approval. A gene editing therapy could follow a similar path for very high-risk patients such as those with heterozygous familial hypercholesterolemia or established atherosclerotic disease who are not at goal despite maximal therapy. Alternatively, authorities could require outcomes data up front for broader primary prevention. The choice will hinge on the totality of Phase 2 and Phase 3 data, including safety, durability, and consistency across subgroups.
Manufacturing and delivery will matter as much as biology. A one-time treatment has to be accessible. That means central manufacturing of messenger RNA and lipid nanoparticles, robust lot release testing, cold chain logistics to infusion sites, and clear care pathways for patient selection and consent. For payers, the pricing model may resemble other one-time genetic medicines with outcomes-based agreements, but the target population here could be much larger. Stakeholders will be modeling long-term savings from avoided heart attacks and procedures against upfront cost.
How it stacks up against today’s options
There is no shortage of ways to lower low-density lipoprotein now. High-intensity statins remain the foundation, with ezetimibe as a low-cost add-on. Monoclonal antibodies that neutralize PCSK9 are highly effective and have outcomes data in secondary prevention, though they require injections every two to four weeks. Inclisiran uses small interfering RNA to silence PCSK9 production and is dosed at day 0, day 90, then every six months thereafter.
Where could VERVE-102 fit if approved? The promise is durability without adherence burden. For patients who cannot tolerate statins or who struggle to keep up with injections, one and done has obvious appeal. On the other hand, the gene editor will come with special counseling, a structured consent process, and post-treatment monitoring. It will not be a first-line option in 2025 or 2026. Even in high-risk patients, physicians will likely use it after conventional therapies have been tried or in those with compelling genetic or clinical reasons to favor a definitive approach.
What you can do now while outcomes mature
You do not need to wait for gene editing to act on cardiovascular risk. Here are concrete steps to take this year:
- Ask your clinician to measure apolipoprotein B and lipoprotein(a) at least once. Apolipoprotein B reflects the number of atherogenic particles and is a better risk marker than low-density lipoprotein alone. For broader context on measurement frameworks, see our biomarker atlas for aging.
- If you have a strong family history of early heart disease, discuss genetic testing for familial hypercholesterolemia and consider a coronary artery calcium scan in midlife to refine risk.
- Work the ladder of current therapies. High-intensity statins first. Add ezetimibe if needed. If not at goal or intolerant, discuss a PCSK9 monoclonal antibody or inclisiran. These are available now and can reduce risk while gene editing evidence matures.
- If you are a candidate for deep prevention due to heterozygous familial hypercholesterolemia or premature coronary disease, ask about ongoing trials and registries in your region. Large centers will know how to screen and refer.
- Keep lifestyle in focus. Lowering apolipoprotein B is the primary goal, but blood pressure, glucose control, sleep, and smoking cessation each compound the benefit. None of these steps conflict with future eligibility for a one-time editor.
The longevity lens
Longevity medicine prizes interventions that are potent, compounding, and simple to maintain. A successful PCSK9 base editor would check those boxes. It could move cardiovascular prevention from compliance management to risk installation. In practical terms, it would turn the familiar advice of take this forever into a single afternoon on an infusion chair followed by routine follow-up. If early data hold and later trials confirm safety, the ripple effects would be large. Primary care pathways would add genotype and particle number testing. Specialty lipid clinics would evolve into procedural hubs for once-per-lifetime cardiometabolic installs. For more on converging cardiovascular and aging biology, see clonal hematopoiesis and heart risk.
There are guardrails. Editing is powerful and should be deployed where absolute risk is high and benefit is clear. Society will need transparent criteria for who gets treated first, how to monitor for rare harms, and how to revisit decisions if new information emerges. None of that diminishes the potential. It only sets the standard for the evidence that must follow. Related trial-readiness themes appear in partial reprogramming enters trials.
What to watch next
By the end of 2025, look for complete dose-escalation data from Heart-2, the start of Phase 2, and a clearer view on durability at the highest well-tolerated dose. In 2026, expect expanded cohorts and perhaps a first look at editing rates in liver biopsy samples from a subset of participants. If pharmacodynamics continue to track with total RNA dose, future protocols may move beyond mg/kg to simpler dose bands that fit clinical practice.
Meanwhile, keep your own risk on a tight leash. Get apolipoprotein B and lipoprotein(a) tested, push your low-density lipoprotein into guideline range with tools that exist today, and follow the VERVE-102 story with a healthy mix of optimism and scrutiny. The line between chronic management and durable prevention is starting to blur. What matters most is arriving at midlife and beyond with a low burden of atherogenic particles in your blood and minimal plaque in your arteries.
The bottom line
April 2025 marked the moment when a one-time cholesterol editor moved from theory to human signal. Fast Track status and the first Heart-2 readout do not guarantee approval, but they change the conversation. Deep low-density lipoprotein reductions after a single infusion are now visible in people, not just in mice or monkeys. If larger and longer studies reproduce that pattern with clean safety, shutting off PCSK9 once could become a new pillar of longevity medicine. Until then, build your own foundation with apolipoprotein B and lipoprotein(a) testing, and use the therapies we already know work while the outcomes evidence for editing catches up.