Thymus Reboot: 2025’s leap from mechanism to clinic
A quiet organ in your chest is emerging as the master switch of immune aging. In 2025, new metabolism data, iPSC thymus breakthroughs, and fresh U.S. funding outline a clinic-ready plan to reset immune age.
The quiet organ that sets your immune age
For decades the thymus sat outside public attention. It is small, it shrinks early in adulthood, and it does its most important work in childhood. Yet the thymus is the schoolhouse for T cells, the white blood cells that patrol for pathogens and cancer. When the thymus is healthy, it graduates a steady stream of naïve T cells, each bearing a new T cell receptor and ready to learn. When the thymus fades, the class size drops. The immune system leans on older memory T cells and the diversity of the T cell receptor repertoire flattens. That shift is a core driver of immunosenescence that shows up as weaker vaccine responses, stubborn infections, and reduced cancer surveillance.
Think of your immune system as a city. Bone marrow is the housing department that builds new recruits. The thymus is the academy that trains them, screens out the reckless, and grants badges. If the academy closes, the city does not collapse right away, but over years the force grows older and less adaptable. Boosting the academy is the most direct way to reverse a citywide trend toward fragility.
In 2025, three lines of progress turned this metaphor into a clinic-ready agenda. First, researchers clarified how metabolism inside the thymus controls its maintenance and decline. Second, stem cell and organoid teams produced convincing human thymus building blocks. Third, U.S. funding momentum created a path to test these ideas in people.
The 2025 shift: metabolism is the throttle on thymic maintenance
This spring a Nature Aging study put a fine point on a long suspected idea. Signals inside thymic stromal cells shape whether the organ remains lean and instructive or slides into fibrous, fatty senescence. Elevating the hormone fibroblast growth factor 21 within thymic stromal cells improved thymic structure in aged mice, increased naïve T cells, and delayed immune aging, while boosting the same hormone from the liver did not deliver the effect. The finding reframes thymic decline as an adjustable metabolic setting inside the organ rather than a passive, whole body fate. It also gives drug developers a near term target in a field that has craved one. See the primary report that paracrine FGF21 delays thymic aging and improves T cell output in old mice in paracrine FGF21 improves thymic function.
Zooming out, the thymus looks like a metabolic balancing act. Anabolic push through insulin like growth factor 1 and mechanistic target of rapamycin tends to accelerate development early in life but can speed senescence later if it remains high. Catabolic and stress responsive pathways, including adenosine monophosphate activated protein kinase and FGF21, can tilt the tissue toward maintenance, cleanup, and quality control. What matters is not a single lever, but the local balance inside thymic epithelial cells and the mesenchyme that supports them. The 2025 data sharpen that point and argue for therapies that act within the thymic niche rather than indiscriminately across the body.
Another 2025 milestone clarified instruction signals that drive human thymic epithelial cell identity. A Science Immunology report showed that type I and type II interferons guide the differentiation of cortical and medullary thymic epithelial lineages and can be harnessed in culture. This matters because good thymus tissue is not just any epithelial sheet. The cortex and medulla teach different lessons to developing T cells. Getting those lessons right in engineered cells is the difference between a tissue that makes useful, tolerant T cells and one that miseducated them.
Put together, spring and summer 2025 turned thymic aging from an abstract inevitability into a concrete, tunable program. Drug development can now aim at the right cell types, with tractable readouts that tell us quickly if the organ is doing its job.
Organoids and iPSC thymus: from sketch to blueprint
If metabolism is the throttle, induced pluripotent stem cells and organoids are the engine parts. Across multiple labs, including groups at Stanford, the Francis Crick Institute, Kyoto University, and the Hubrecht Institute, teams reported human induced pluripotent stem cell derived thymic epithelial cells that look and act like their in vivo counterparts. In preclinical systems, these cells support the stepwise development of human T cells from double negative to double positive to single positive stages, and they express markers of cortical and medullary identity, including the autoimmune regulator Aire in the right context. The headline is not just that we can make thymic epithelial cells. It is that we can make the right kinds, in the right proportions, and they can perform the essential tests of positive and negative selection.
Organoid advances filled in the rest of the picture. Researchers can now grow structured, multicellular thymic organoids that include epithelium, mesenchyme, and vasculature mimics. These organoids educate developing T cells in vitro and, in animal models, after transplantation. That dual ability is critical. In vitro systems allow fast iteration on design, potency assays, and quality control, while in vivo implantation shows whether the constructs can vascularize, persist, and produce a measurable stream of new T cells.
Companies have begun to translate these pieces. Thymmune Therapeutics is building scalable induced pluripotent stem cell thymic cell products intended to restore thymic function in both rare immunodeficiencies and aging. Tolerance Bio is developing allogeneic, off the shelf thymus cell therapies and companion drugs that modulate thymic biology. Smart Immune is advancing ex vivo generated T cell precursors that seed the thymus and accelerate reconstitution after stem cell transplant. These are complementary strategies that share one thesis. If you restore the academy, the immune system can replace a patchwork of temporary fixes with durable immune competence.
A credible path into humans: three cohorts, one playbook
Every new regenerative therapy needs a safe, stepwise route into the clinic. Thymus rejuvenation has a head start because the U.S. Food and Drug Administration already approved a one time thymus tissue therapy for children born without a thymus. That product, marketed as Rethymic, established both the clinical need and the regulatory precedent for thymus based immune reconstitution. It also taught the field about time to benefit, infection risk management, and how to monitor thymic output post implant. See the agency and sponsor summary of the therapy in FDA approval of a thymus tissue therapy.
Using that foundation, first in human studies for engineered thymus therapies can proceed through three expanding cohorts:
-
Ultra rare congenital athymia and severe thymic hypoplasia. These patients have the most to gain and the clearest risk benefit profile. Primary endpoints should focus on thymic output and functional protection from infection. Because these children are often on immunoglobulin replacement and antimicrobial prophylaxis, protocols should detail how to taper support as de novo T cell output rises. Safety monitoring must include automated assays to catch early signs of graft versus host disease or miseducation driven autoimmunity.
-
Post chemotherapy or post hematopoietic stem cell transplantation immune rebuild. Many cancer survivors and transplant recipients live with lingering lymphopenia and limited naïve T cell pools for months to years. An engineered thymus graft or a thymus seeding cell therapy could compress that timeline. Trial designs should stratify by age, prior radiation, and thymic volume on imaging, since the native scaffold varies. Endpoints can include time to reach a predefined naïve T cell threshold, time to successful vaccination with new antigens, and rates of clinically significant infections over the first year.
-
Midlife immune reset for prevention. Adults in their 40s, 50s, and 60s who show low thymic output and poor vaccine responses despite otherwise good health represent the first preventive cohort. Here, small implants or transient boosters that tilt thymic metabolism and replace missing epithelial niches may be sufficient. Trials should enroll those with clear baseline deficits to maximize signal and minimize exposure in already robust individuals.
Across all cohorts, build trials with conservative dosing, rigorous stopping rules, and long term follow up. Immunology moves in months, but autoimmunity unfolds over years. The playbook must respect both.
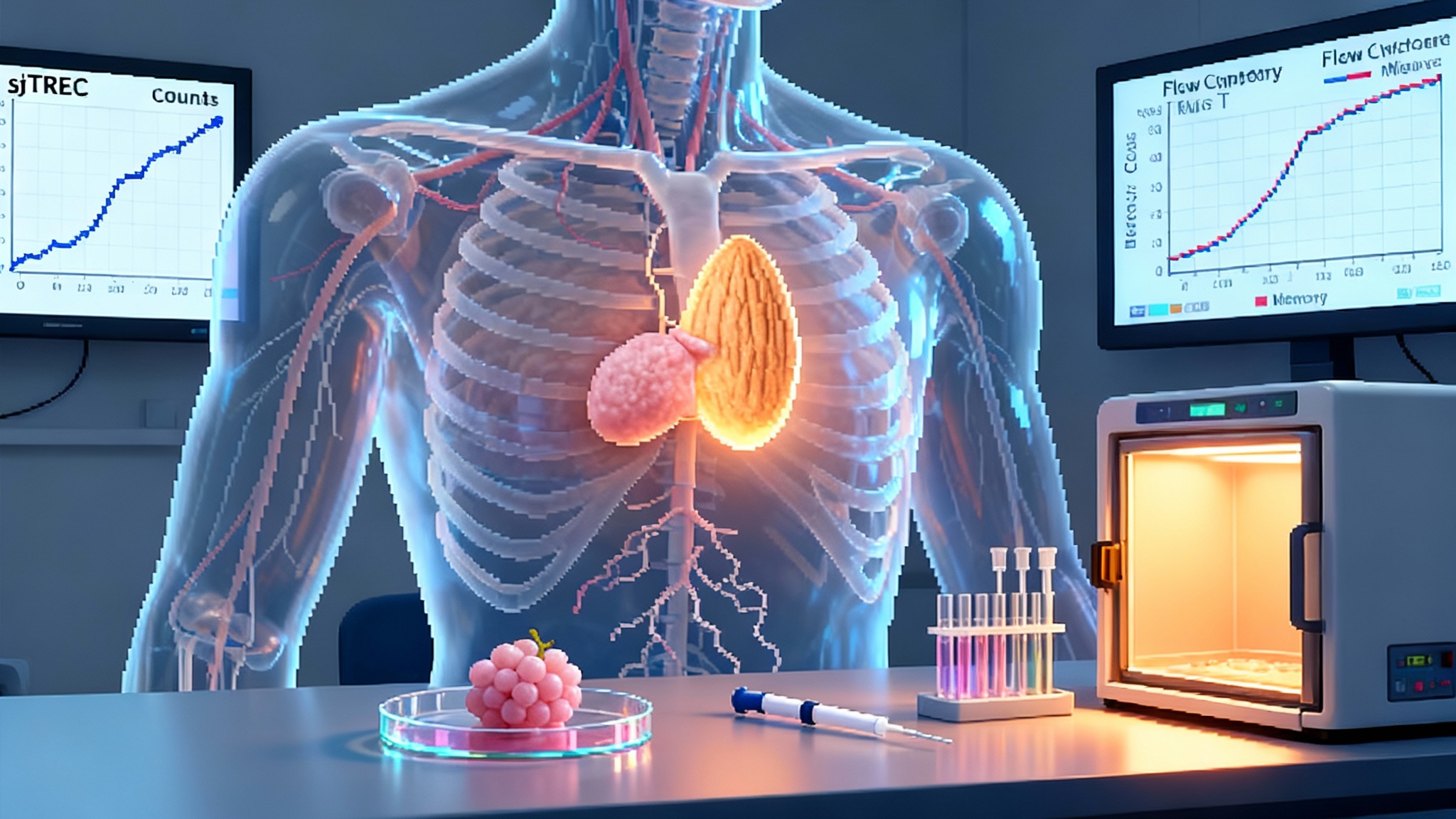
What to measure: biomarkers that prove a true reboot
To escape hand waving claims, thymus rejuvenation needs objective markers of new T cell production and useful immune function. Four categories can anchor a common measurement set:
-
Direct thymic output. Signal joint T cell receptor excision circles, called sjTRECs, are small pieces of DNA left over when T cells rearrange their receptors in the thymus. Because they do not replicate during cell division, their abundance in peripheral blood reflects the number of new thymic graduates entering the circulation. Report sjTREC counts per microliter and normalize to total T cell counts to avoid dilution by expansion of older cells.
-
Composition and diversity. Flow cytometry can quantify naïve to memory ratios across CD4 and CD8 compartments, with CD45RA and CD62L markers for naïve cells and CD45RO and CCR7 for memory subsets. Track CD31 on naïve CD4 cells as a marker of recent thymic emigrants. Sequence T cell receptors to measure repertoire richness and evenness using diversity indices like Shannon or Simpson. A recovering thymus should increase both the proportion of naïve cells and the diversity of receptors.
-
Functional response. Prospective vaccine challenges provide the cleanest test. Choose at least one novel antigen platform such as a recombinant protein or mRNA vaccine and measure neutralizing titers, T cell interferon gamma release, and memory durability at three and six months. For broader context on vaccine strategies in aging, see the CMV vaccine that could rewire aging. Layer in clinical endpoints that matter to patients, including time to first respiratory infection and days of antibiotics per year.
-
Anatomy and microstructure. Magnetic resonance imaging can quantify thymic volume and fat fraction without ionizing radiation. When surgery allows, single cell RNA sequencing and histology from explanted or biopsy material can confirm cortical and medullary organization, Aire expression in medulla, and vascularization. In cell therapy manufacturing, release assays should include antigen presentation capacity and cytokine responsiveness that mirror in vivo maturation states.
For teams building shared cores and standards, the SenNet’s 2025 biomarker atlas is a useful reference for harmonizing assays and endpoints.
These markers keep teams honest. If a candidate raises total T cells but not sjTRECs, it is likely just expanding old clones. If it raises sjTRECs but fails vaccine responses, the organ may be producing but not educating correctly.
Safety, ethics, and regulation: the real risks and how to manage them
Engineered thymus therapies carry specific hazards. The first is tumorigenicity from residual pluripotent cells in an induced pluripotent stem cell derived product. This is addressable with rigorous differentiation protocols, suicide gene safeguards, and release testing that enforces no detectable pluripotency markers. The second is autoimmunity if the engineered tissue fails negative selection or presents self antigens abnormally. This requires careful control of Aire positive medullary epithelial cell content, appropriate expression of tissue restricted antigens, and long term monitoring for organ specific autoantibodies and clinical signs. For complementary tolerance strategies, see engineered Tregs after the Nobel.
Alloimmunity is another design choice. Autologous induced pluripotent stem cell products avoid rejection but add time and cost. Allogeneic, off the shelf products improve access and standardization, but they must be either immune evasive or matched carefully. Because thymic epithelial cells are professional educators rather than professional antigen presenting cells, the acceptable threshold for matching may be different from that of blood or solid organ transplants. Early trials should include donor specific antibody screening and a plan for short course immunosuppression at implant.
From a regulatory view, thymus therapies are well suited to the U.S. Food and Drug Administration’s Regenerative Medicine Advanced Therapy pathway, which offers guidance and potential expedited review for serious conditions with preliminary clinical evidence. The precedent of a licensed thymus tissue therapy helps. So does the field’s growing consensus on potency assays, including in vitro education of T cells and in vivo sjTREC rise after implant. Manufacturing scale and consistency will be as decisive as biology, which is why alignment with agencies on release specifications in phase 1 is worth the effort.
Funding momentum and why it matters
Ambition rises when dollars meet discipline. Over the past year, U.S. programs have pushed immune aging from whiteboard to roadmap. The Advanced Research Projects Agency for Health funded a multiyear Thymus Rejuvenation effort led by Thymmune Therapeutics to build induced pluripotent stem cell thymic epithelial cells at scale and to test them in models of congenital athymia and aging. ARPA H’s broader healthspan program, PROSPR, is organized around translating aging biology into practical interventions with regulators at the table. On the private side, venture backed companies focused on thymus biology have been able to hire manufacturing talent and set up the quality systems that make clinical grade cell products possible.
Why does this matter to readers outside the lab or boardroom? Because a working thymus reboot would multiply the value of almost every other medical advance. Better cancer vaccines, more durable responses to respiratory pathogens, fewer hospitalizations for opportunistic infections, and more treatment options for patients who previously could not tolerate immune based therapies all flow from a healthier T cell factory. It is a lever that moves many stones at once.
A practical build order: 2025 to 2030
Here is a concrete plan for getting from today’s preclinical wins to population scale immune age reversal by the end of the decade.
2025 to mid 2026
- Finalize good manufacturing practice protocols for induced pluripotent stem cell derived thymic epithelial cells and composite organoids. Lock potency assays that correlate with in vivo education, including induction of CD69 high, T cell receptor rearranged thymocytes in co culture.
- Complete biodistribution and tumorigenicity studies in large animals. Include sensitive assays for residual pluripotency and for ectopic tissue formation at off target sites.
- File early regulatory packages and seek Regenerative Medicine Advanced Therapy and orphan designations where applicable. Align on release criteria, sjTREC based pharmacodynamic markers, and imaging schedules.
Late 2026 to 2027
- Launch phase 1 trials in congenital athymia and severe thymic hypoplasia. Primary endpoints are safety and de novo T cell output measured by sjTRECs, with secondary endpoints of infection rates and vaccine response. Include at least 12 months of follow up.
- Start a small post chemotherapy or post transplant cohort focused on time to immune reconstitution. Power the study to detect a clinically meaningful reduction in days to reach a naïve CD4 threshold and successful vaccination against one new antigen.
- Iterate manufacturing for cost, including moving from bespoke implants to modular constructs that fit varied anatomies.
2028
- Expand to multi site trials with standardized imaging and immune monitoring cores. Adopt platform protocols that allow multiple companies to test implants or seeding cells against shared endpoints. This reduces duplication and speeds learning.
- Begin the first preventive midlife immune reset trial with tight inclusion criteria and a pragmatic design that captures real world events like respiratory infections and days of work missed.
- Publish a harmonized biomarker framework that regulators and payers can use to evaluate benefit, including sjTREC thresholds, naïve to memory ratios, and repertoire diversity cutoffs that define success. For assay alignment, the SenNet’s 2025 biomarker atlas offers a useful starting point.
2029 to 2030
- If safety holds and efficacy is clear, move to pivotal studies in the highest need populations. Pursue adaptive licensing models that allow early, narrow use with mandatory registries.
- Build reimbursement around avoided hospitalizations and vaccine failures, not just cell product cost. Create risk sharing agreements that tie payment to durable sjTREC rises and functional markers at one year.
- Stand up a national thymus registry to track long term outcomes, autoimmunity signals, and effectiveness across diverse populations. Use it to refine indications and dosing.
What clinicians, investors, and policymakers can do now
- Clinicians: start measuring sjTRECs and naïve to memory ratios in older patients with recurrent infections or poor vaccine responses. These tests identify who might benefit from early trials and create baselines for future care.
- Hospital systems: prepare for cell therapy logistics even for small implants. Operating room time, imaging, and immune monitoring infrastructure will be the rate limiters, not just the cell product.
- Investors: prioritize platforms with clear potency assays and a credible path to scale. Manufacturing, quality control, and release testing are not back office tasks, they are the product.
- Policymakers: keep aging biology translation programs focused on shared endpoints and open registries. Funding that ties industry, academics, and regulators to the same markers will reduce trial and error and speed approvals.
The bottom line
The thymus is the master switch of immunosenescence because it controls the supply of new, trainable T cells. In spring and summer 2025, we learned that the organ’s decline is not a one way slide. Local metabolic programs can be tuned to preserve function, and human engineered thymus tissues can now reproduce the lessons that make T cells both diverse and self tolerant. With credible precedents and growing funding, a careful, staged rollout from rare disease to recovery to prevention is within reach. If we execute the plan with the right biomarkers and safeguards, by the turn of the decade a thymus reboot could become a routine part of maintaining health, not a moonshot.